Introduction
Chronic, hard-to-heal wounds, including diabetic foot ulcers (DFUs), venous leg ulcers (VLUs), and pressure ulcers (PUs) remain a significant challenge for wound care specialists and healthcare systems. These wounds are frequently refractory to standard therapies and may persist for months or years before resolution. Their protracted course imposes a substantial economic burden on healthcare resources while also diminishing patients' quality of life and functional independence.1 The increasing prevalence of chronic wounds, particularly among older adults and individuals with comorbidities such as diabetes, obesity, or peripheral vascular disease, underscores the need for more effective interventions.1
DFUs develop in approximately 34% of patients with diabetes during their lifetime and represent the leading cause of non-traumatic lower limb amputations worldwide.2 In addition to DFUs, VLUs and PUs constitute major global health challenges. VLUs affect up to 1–3% of the adult population, are notoriously chronic, and account for significant morbidity and healthcare costs.3 Pressure ulcers, similarly, are highly prevalent in hospitalized and long-term care patients, with reported incidence rates ranging from 5–15%, and are associated with increased risk of infection, prolonged hospitalization, and reduced quality of life.4,5 Together, these chronic wounds represent a substantial clinical and economic burden, underscoring the need for effective and accessible wound care strategies.
Cold atmospheric plasma (CAP) has emerged as a promising adjunctive therapy for chronic wounds. CAP is an ionized gas that operates at near-ambient temperature and delivers a mixture of reactive oxygen and nitrogen species, neutral particles, electromagnetic fields, and low-level ultraviolet radiation.6 These reactive species exert multifaceted effects: they promote wound healing by stimulating cytokine release, enhancing angiogenesis, and supporting cellular proliferation, while simultaneously exhibiting broad-spectrum antimicrobial activity. In bacterial and fungal cells, CAP induces oxidative stress and disruption of macromolecules, including DNA, leading to pathogen inactivation regardless of species or antibiotic resistance profile. This renders CAP particularly attractive in the current era of rising antimicrobial resistance.7
Preliminary studies suggest encouraging outcomes with CAP for chronic, hard-to-heal wounds.8-14 Further clinical data are required to confirm its safety and efficacy and to establish its role in wound management. This study evaluates the plasma care® device, which incorporates a plasma care® spacer and is designed as a compact, user-friendly medical device for wound treatment. The device uses ambient air and electrical energy to generate CAP, which can be applied as a standalone therapy or in conjunction with other wound care modalities. By inactivating bacteria and fungi irrespective of species or antibiotic resistance status, plasma care® has the potential to provide an effective adjunctive approach for patients with long-standing, treatment-resistant ulcers.
Methods and materials
Study design, population, and overview
The potential application of CAP in the treatment of difficult-to-heal wounds was assessed in this prospective case series. Plasma care® treatment was administered twice a week for 4 weeks to participants who met the inclusion criteria. The standard of care for DFUs included offloading, sharp debridement, and maintaining an appropriately moist wound healing environment. Compression therapy, exudate control, and reducing wound bed bacterial load were the standard of care procedures for VLUs. Debridement, off-loading, reducing bacterial load and the promotion of moist wound healing were the standard of care procedures for PUs.
The primary objective of the study was to determine the proportion of hard-to-heal DFUs and VLUs that achieved >40% wound area reduction at 4 weeks, as well as the proportion of pressure ulcers that achieved >10% percent area reduction in 4 weeks.
The study population was drawn from individuals attending wound clinics for the treatment of chronic wounds. These individuals were selected from the general population. A total of 12 subjects were enrolled in the study.
Study procedure
The study consisted of two main phases: the screening phase and the treatment phase. The screening phase involved a series of assessments to determine eligibility for participation. If individuals met the eligibility criteria, they proceeded to the treatment phase.
During the screening visit, the investigator or their representative obtained an IRB-approved written informed consent from the participants before conducting any specific procedures outlined in the protocol. At this visit, the investigator selected a specific ulcer, known as the index ulcer, for each participant. The index ulcer had to be at least 4 cm away from any other open ulcer. If a participant had multiple ulcers, the investigator chose the largest one that met the protocol's eligibility criteria as the index ulcer.
The purpose of the screening visit was to determine whether participants were eligible to enter the treatment phase of the study. Those who met the eligibility criteria began the treatment phase on the same day as the screening visit or at a later date, known as treatment visit 1 (TV1).
During the treatment phase, which lasted for 4 weeks, eligible participants received CAP in addition to standard-of-care treatment which consisted of wound cleansing, debridement as needed, a 4x4 gauze soaked with wound cleanser for 10 minutes, two-layer compression wrap or offloading depending on the wound type. CAP was administered twice weekly. The effectiveness of the treatment was assessed weekly through the investigator's evaluation of ulcer healing and measurements of ulcer size using the digital photographic device MolecuLightDX™, which provides precise digital wound measurements.15 It can also detect bacterial loads at the chronic inhibitory bacterial load (CIBL).16
TABLE 1 Inclusion and exclusion criteria
| Inclusion criteria At least 18 years old. Presence of a diabetic foot ulcer, Wagner 1 or 2 grade, extending at least through the dermis or subcutaneous tissue and may involve the tendon or muscle provided it is below the medial aspect of the malleolus. Presence of a full-thickness venous leg or pressure ulcer without exposure of underlying tissues and free of non-viable tissue. Index ulcer (i.e., current episode of ulceration) has been present for greater than 4 weeks prior to the initial screening visit. Study ulcer size is a minimum of 0.75 cm2 and a maximum of 5 cm2. at the first treatment visit for diabetic foot ulcers and a minimum of 2 cm2 and a maximum of 20 cm2 for venous leg and pressure ulcers. Adequate circulation to the affected extremity (if applicable) as demonstrated by a transcutaneous oxygen measurement (TCOM) or a skin perfusion pressure (SPP) measurement of ≥30 mmHg, or an Ankle Brachial Index (ABI - measure of blood flow to the ankle) between 0.7 and <1.3 within 3 months of the first screening visit. Females of childbearing potential must be willing to use acceptable methods of contraception (birth control pills, barriers, or abstinence). The subject understands and is willing to participate in the clinical study and can comply with weekly visits and the follow-up regimen. The subject has read and signed the IRB-approved Informed Consent Form before screening procedures are undertaken. Exclusion criteria Study ulcers(s) deemed by the investigator to be caused by a medical condition other than diabetes or venous disease or related to a pressure injury. A surgery for operative debridement or revascularization is planned for the ulcer to be treated. Index ulcer has a history of cancer or, in the opinion of the investigator, is suspicious for cancer and should undergo an ulcer biopsy to rule out a carcinoma of the ulcer. Subjects with a history of more than 2 weeks of treatment with immunosuppressants (including systemic corticosteroids > 10mg daily dose), cytotoxic chemotherapy, or application of topical steroids to the ulcer surface within 1 month prior to the first screening visit, or who receive such medications during the screening period, or who are anticipated to require such medications during the course of the study. History of radiation at the ulcer site. Subject who cannot have routine dressing changes. Subject who cannot adhere to strict offloading according to protocol standards in the opinion of the investigator. Presence of any condition(s) which seriously compromises the subject's ability to complete this study or has a known history of poor adherence with medical treatment. Active treatment of infection anywhere in the body with IV antibiotics, at screening and baseline. Suspected or confirmed signs/symptoms of gangrene on any part of the affected limb. Known osteomyelitis or bone infection of the affected foot or leg as verified by diagnostic imaging within 30 days prior to enrollment. Subject is pregnant or breastfeeding. Study ulcer with a history of treatment with hyperbaric oxygen, growth factors or other biologic treatments, or a cellular or tissue-based product (CTP) within 30 days of enrollment. Subject has a known or suspected allergy to products under study. Concurrent disease or drugs known to induce severe photosensitivity of the skin, such as porphyria. |
The plasma care® device used in this study is a portable dielectric barrier discharge (DBD) cold atmospheric plasma generator designed to produce reactive oxygen and nitrogen species directly from ambient air. It uses a floating electrode configuration and an integrated spacer that standardizes the 2–5 mm treatment distance to ensure reproducible delivery. The device operates at temperatures below 40°C, preventing thermal injury, and delivers treatment in 2-minute cycles per application zone, consistent with prior clinical research involving plasma care®. Unlike gas-dependent CAP systems using argon or helium, plasma care® relies solely on ambient air, making it more practical and accessible for outpatient wound-care environments.
Statistical analysis
For all categorical variables (gender, wound type, race, ethnicity) counts and percentages were calculated. For quantitative demographic information (patient age, initial wound area, and wound age) summary statistics including mean, minimum, maximum, and median were calculated. A 95% confidence interval was calculated for mean wound area cm2 for all patients.
To assess the primary endpoint, the percent area reduction (PAR) was calculated for each patient. If A1 is defined as the wound area at treatment visit 1, and A2 is defined as the wound area at treatment visit 9 (or end of study), then PAR can be calculated using the formula below:
Results
Demographic information was collected from the 12 patients during enrollment and analyzed using summary statistics. The mean patient age was 63.08 years old, with six male (50%) and six female (50%) patients. Ten patients were Caucasian (83%), one patient was American Indian/Alaskan Native (8.3%), and one patient was Black/African American (8.3%). None of the patients enrolled were Hispanic/Latino. To assess the primary endpoint, wound type was determined for the index ulcer. Five venous leg ulcers (41.6%), six diabetic foot ulcers (50%), and one pressure ulcer (8.3%) were included in the study. These patients had a mean initial wound area of 3.05 cm2 and wound age of 79.7 weeks. Two patients withdrew (16.7%), one patient at treatment visit 7 and one patient at treatment visit 9.
Figure 1 shows a 95% confidence interval of the mean wound area for all patients. Due to the small sample size, the width of the interval is large. Patients saw an overall mean percent area reduction of 21%.
FIGURE 1 95% Confidence interval of mean wound area (cm2).
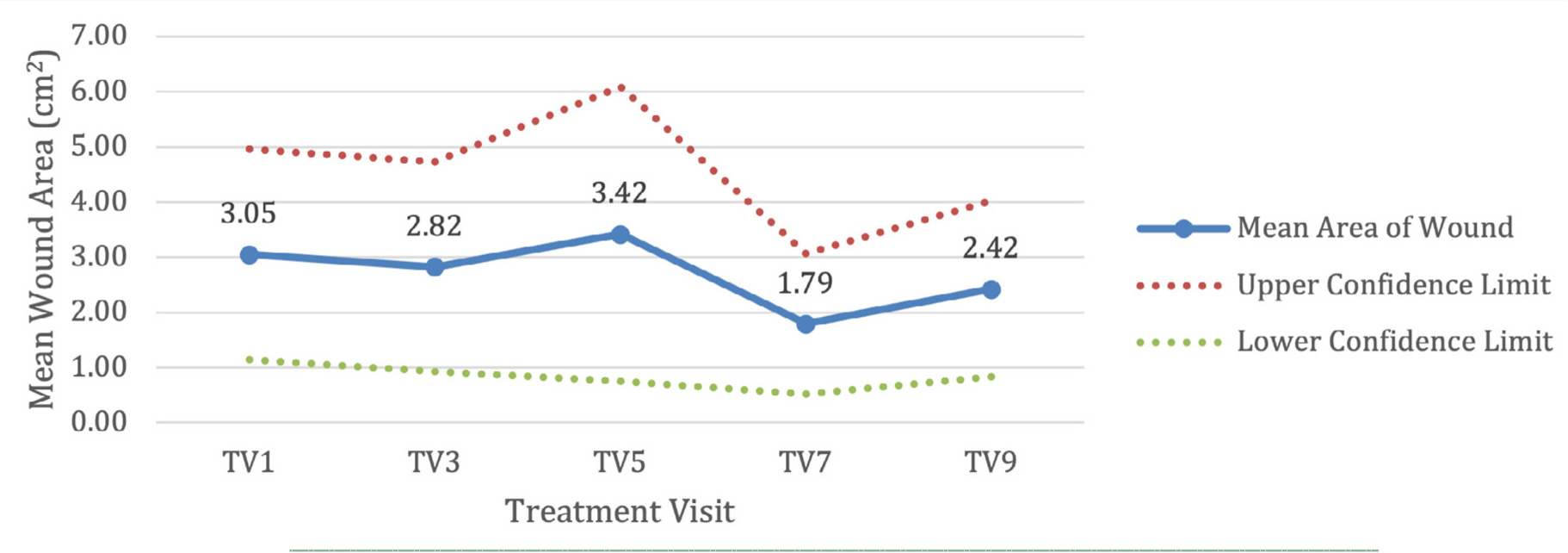
Table 2 provides further information on wound healing by wound type to assess the primary objective. Of the DFU patients who had PAR >40%, one patient healed (PAR = 100%) at treatment visit 7.
TABLE 2 Percent area reduction by wound type. Count (Percent).
| Wound type | PAR >40% | PAR <40% |
|---|---|---|
| Diabetic foot ulcer | 3 (50%) | 3 (50%) |
| Venous leg ulcer | 3 (60%) | 2 (40%) |
| PAR >10% | PAR <10% | |
| Pressure ulcer | 0 | 1 (100%) |
The proportion of DFUs that achieved >40% wound area reduction at 4 weeks is 50%, while the proportion of VLUs that achieved >40% wound area reduction at four weeks is 60%. The pressure ulcer included in the study did not achieve a PAR >10% at 4 weeks.
Although quantitative cultures were not collected, wound bacterial burden was qualitatively monitored using MolecuLightDXTM fluorescence imaging, which detects regions of CIBL. Across the twelve treated ulcers, eight demonstrated reductions in fluorescence intensity over the 4-week period, consistent with a decrease in bacterial burden. No patient developed a new clinical infection, and aside from one case of transient periwound erythema that resolved without intervention, no infection-related adverse events occurred. These observations align with CAP's known antimicrobial activity, although future work incorporating quantitative microbiology would further strengthen these findings
Case presentation: DFU
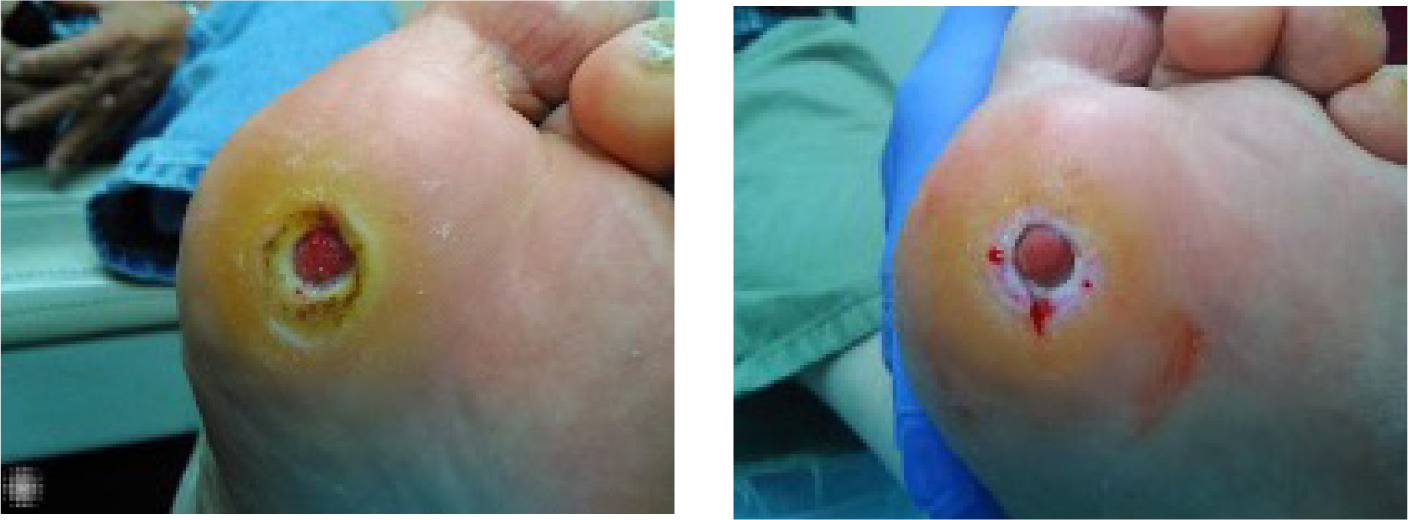
A 69-year-old Caucasian male with a past medical history of hypertension, type 2 diabetes mellitus, hyperlipidemia, and benign prostatic hyperplasia presented with a recurrent diabetic foot ulcer (DFU) on the plantar surface of the left foot, measuring 1.68 cm2. The patient reported a previous ulcer at the same location that had closed but subsequently re-opened. He is retired and has been compliant with prescribed management plans, including strict adherence to offloading since the recurrence of the wound.
On physical examination, the patient appeared well, with an open plantar ulcer surrounded by abundant periwound callus. There were no clinical signs of infection in the wound bed or adjacent tissues. Despite adherence to standard wound care protocols and receiving several advanced wound care therapies, the ulcer failed to progress toward healing. Given the chronic refractory nature of the wound, the patient was enrolled in the case series and underwent CAP treatment.
The wound progress is shown in Figure 2.
FIGURE 2 Screening vs treatment visit 9/eos Baseline MoleculightDx photo (left), fifth-week follow-up photo (right)
Case presentation: pressure ulcer
A 57-year-old female presented with a chronic, nonhealing pressure ulcer of 6 years' duration (Figure 3). Her past medical history was notable for paraplegia following a motor vehicle accident 28 years earlier, which resulted in permanent wheelchair dependence. To manage complications associated with her paraplegia, she had a colostomy and a suprapubic catheter in place.
FIGURE 3 Treatment visit 1(left) and end of study visit (right).
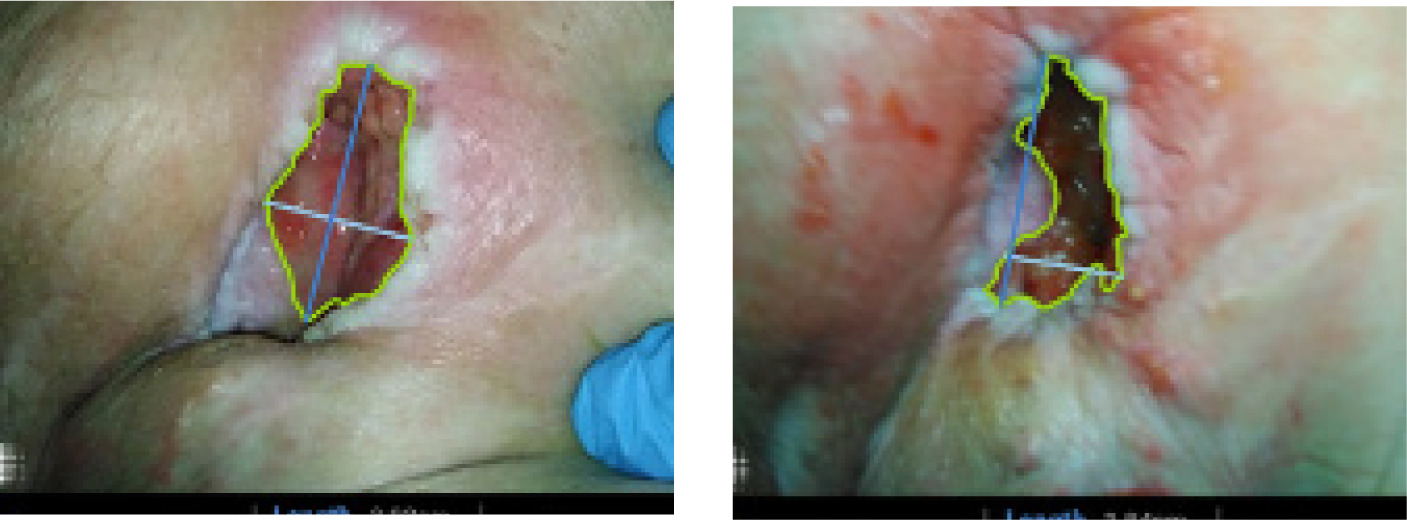
The patient's social history was significant for unemployment and prior tobacco use, both of which may negatively impact wound healing. She also had several additional comorbid conditions, including chronic pressure ulcers, complex pain disorder, anxiety, depression, and recurrent osteomyelitis since 2019. These overlapping medical and psychosocial factors contributed to the chronicity and complexity of her wound, as well as significant functional impairment and diminished quality of life.
Given the prolonged course of her ulcer, the history of underlying osteomyelitis, and the numerous intrinsic and extrinsic risk factors for impaired healing, this case highlights the clinical challenges in managing advanced pressure injuries in patients with longstanding paraplegia.12
The pressure ulcer case demonstrated mild qualitative improvement despite the absence of measurable area reduction. Over the course of treatment, the wound bed developed healthier granulation tissue, and the surrounding inflammation decreased slightly. Although these changes did not translate into substantial PAR within the 4-week window, they suggest an early biologic response in a highly complex, long-standing pressure injury complicated by multiple comorbidities.
Case presentation: VLU
A 62-year-old Caucasian male with a medical history of chronic peripheral venous insufficiency presented with a venous leg ulcer on the lateral aspect of his left lower leg. The ulcer had developed spontaneously without preceding trauma, had been present for 6 weeks at the time of enrollment, and measured 2.13 cm2 (Figure 4, left picture). On examination, the wound was shallow with irregular borders, surrounded by hyperpigmented skin and mild periwound redness consistent with venous stasis changes. There were no signs of acute infection.
FIGURE 4 Treatment visit 1 (left) and end of treatment (right)
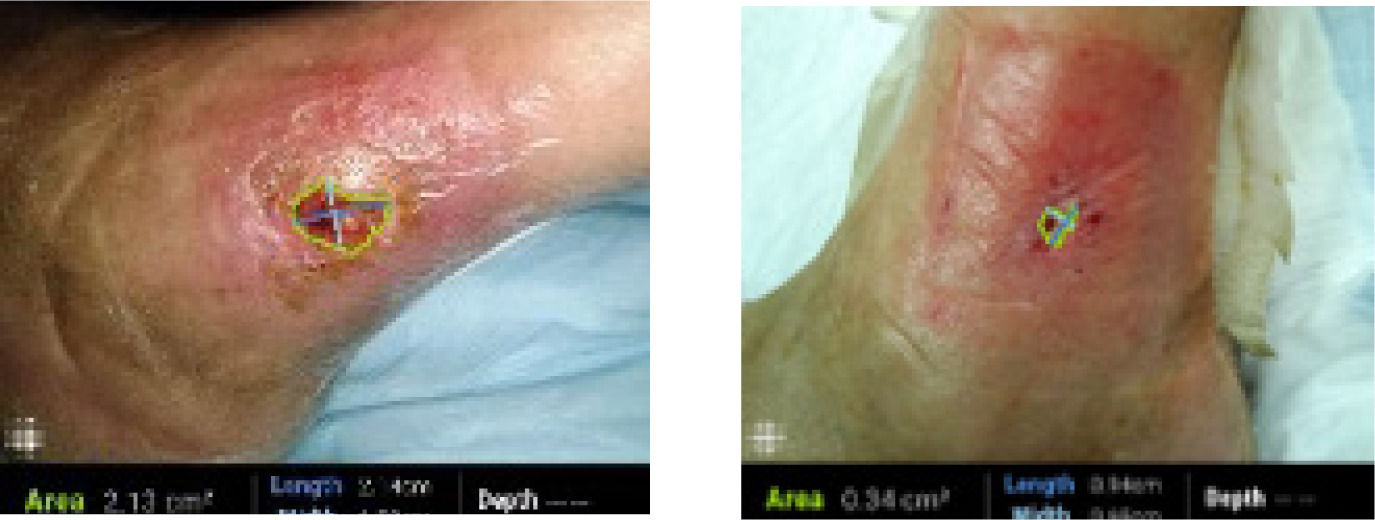
The patient was enrolled in the study and treated with cold atmospheric plasma in addition to standard compression therapy. After 4 weeks, the ulcer decreased in size to 0.34 cm2 (Figure 4, right picture), reflecting an 84% reduction in wound area. The wound bed exhibited healthy granulation tissue, with only modest improvement in periwound inflammation.
Discussion
The results of this prospective case series demonstrate that CAP, when applied as an adjunct to standard wound care, may enhance healing outcomes in patients with chronic, hard-to-heal ulcers. Overall, participants achieved a mean wound area reduction of 21%, with 50% of DFUs and 60% of VLUs reaching the predefined endpoint of >40% PAR at 4 weeks. Although the single pressure ulcer case did not demonstrate measurable improvement, these findings are consistent with the growing body of evidence supporting CAP as a promising therapeutic modality in wound management.
The thresholds of >40% PAR for DFUs and VLUs and >10% PAR for pressure ulcers were selected based on established patterns of early healing documented in the chronic wound literature. In DFUs, early wound area change has been repeatedly validated as a powerful predictor of long-term healing. Sheehan et al demonstrated that the percent change in DFU area over a 4-week period is strongly associated with complete closure at 12 weeks, establishing early PAR as a reliable prognostic indicator.17 Lavery et al later confirmed that early wound area progression at 1 and 4 weeks similarly predicts healing at 16 weeks after partial foot amputation.18 For VLUs, Phillips et al reported that a 30–40% reduction over several weeks is a meaningful threshold that correlates with eventual wound closure.19 Pressure ulcers, however, heal at a substantially slower rate due to impaired mobility, perfusion deficits, and heavier systemic comorbidities. In this population, Thomas et al found that even modest early changes, such as a 10% reduction at 4 weeks, represent clinically relevant improvement.20
PAR at 4 weeks was selected instead of complete wound closure because PAR is a validated surrogate marker of healing trajectory and is particularly suited to short-duration, early-phase clinical studies. Complete closure often requires several months of follow-up and is heavily influenced by long-term adherence, comorbidity burden, offloading, and perfusion status, making it too distant an outcome for a 4-week exploratory case series. Early PAR, in contrast, has been repeatedly shown to reflect meaningful therapeutic response. Sheehan et al demonstrated that the percent change in DFU area at 4 weeks is a strong predictor of eventual healing,17 while Lavery et al confirmed that early wound progression at 1 and 4 weeks predicts final outcomes at 16 weeks.18 Phillips et al also reported that early changes in wound area are prognostic for healing in venous ulcers.19 Armstrong and Lavery also highlighted the importance of serial wound measurements in assessing risk and predicting outcomes in diabetic foot care.21 Given CAP's expected early biologic effects on angiogenesis, microcirculation, inflammation, and bacterial burden, PAR provides a more sensitive and appropriate endpoint than complete closure within a 4-week timeframe.
Clinical relevance and comparison to prior studies
Chronic wounds are notoriously difficult to treat, with DFUs, VLUs, and pressure injuries contributing significantly to morbidity, healthcare costs, and reduced quality of life. Standard interventions – offloading, compression therapy, and debridement – remain foundational, but their limitations highlight the need for adjunctive strategies. In this study, CAP demonstrated bactericidal activity, safety, and tolerability, consistent with prior studies on CAP.
Isbary and colleagues conducted the first prospective randomized controlled trial using cold argon plasma on chronic wounds and observed significant reductions in bacterial load without adverse tissue effects.7 Subsequent studies confirmed CAP's antimicrobial activity and reported improvements in wound closure rates.8,9 A 2020 randomized clinical trial of CAP for the treatment of DFUs, found enhanced healing rates compared with standard care.11 A 2021 mechanism of action study demonstrated improved microcirculation and angiogenesis following repetitive CAP application, suggesting mechanistic support for its role in tissue repair.12 The findings in this case series align with these reports.
It is theorized that bacterial deactivation, enhanced angiogenesis, and modulation of inflammatory responses likely contributed to wound size reduction. Patients also reported the treatment as painless, supporting prior observations that CAP is well tolerated even in comorbid populations with fragile skin and limited healing capacity.
Mechanisms of action
The therapeutic potential of CAP derives from its production of reactive oxygen and nitrogen species (RONS), electromagnetic fields, and low-level ultraviolet radiation. These factors act synergistically to modulate wound healing pathways: RONS induce transient oxidative stress that stimulates angiogenesis and cytokine release, while exerting antimicrobial effects by damaging microbial membranes, DNA, and proteins. In vitro and in vivo studies further suggest CAP influences keratinocyte proliferation, immune modulation, and upregulation of antimicrobial peptides. Such pleiotropic effects may explain the accelerated healing seen in treatment-resistant ulcers.13,14
Limitations
Several limitations must be acknowledged. The sample size was small (n=12), limiting statistical power and generalizability. The heterogeneity of wound types introduces variability that complicates interpretation, particularly as only one PU was included. The short follow-up duration (4 weeks) may underestimate long-term benefits or recurrence rates. Additionally, while the plasma care® device was well tolerated, one participant developed transient periwound erythema, underscoring the need for continued safety monitoring. Finally, although bacterial deactivation was observed, quantitative microbiological outcomes were not systematically measured, which could strengthen conclusions about CAP's antimicrobial efficacy.
Future directions
Larger, multicenter randomized controlled trials are needed to confirm these preliminary results, establish optimal treatment frequency, and evaluate long-term healing outcomes. Comparative studies against other advanced wound therapies would further clarify CAP's position within the therapeutic armamentarium. Mechanistic investigations into CAP's effects on host immune responses, angiogenesis, and biofilm disruption will help refine patient selection and treatment protocols.
Conclusion
This study adds to the growing evidence that CAP is a safe, well-tolerated, and potentially effective adjunct for chronic wound management. By combining broad-spectrum antimicrobial action with pro-healing biological effects, CAP addresses both the microbial and host-driven barriers to healing. Although larger trials are warranted, CAP represents a promising addition to the therapeutic landscape for DFUs, VLUs, and other refractory wounds.