Introduction
Although many advanced treatment options exist, fewer than half of chronic wounds close within a 12-week period.1 Patients suffering from chronic wounds often face reduced quality of life, functional limitations, and, in some cases, severe complications, such as lower extremity amputation.2 A key factor contributing to delayed healing is the presence of bacteria. When bacterial concentrations exceed 104 colony-forming units per gram (CFU/g) of tissue, wound healing is impaired.3-5 A randomized controlled trial involving 228 patients demonstrated that bacterial loads above this threshold were strongly associated with poor healing outcomes.3 Similarly, other investigations have reported delayed wound healing when bacterial counts surpassed 105 CFU/g.4,6 Efforts to reduce clinically significant bacterial burden are an important strategy to enhance wound closure.
To promote healing and prevent infection, clinicians must be able to identify and effectively manage bacterial bioburden. Unfortunately, the clinical signs and symptoms (CSS) of infection are not reliable in detecting bacteria in nonhealing wounds.5 The use of topical antimicrobials and antibiotics based solely on CSS is haphazard at best.7 Moreover, patients with chronic wounds are prescribed antibiotics at much higher rates than those without wounds.8 A post hoc analysis of a 350-patient clinical trial demonstrated that most of the Centers for Disease Control and Prevention pathogens of concern are located at the margin of the wound.9
A pivotal trial on the detection of clinically significant levels of bacteria in nonhealing wounds, Fluorescence Imaging Assessment and Guidance (FLAAG) (ClinicalTrials.gov identifier: NCT03540004), showed that CSS identify clinically significant bacterial loads in 15-20% of wounds. The same study demonstrated that adding real-time fluorescence imaging increased the identification of bacteria at levels greater than 104 CFU/g by 4 to 7 times, leading to better treatment guidance far superior to CSS alone.5 The detection of bacteria was more pronounced in dark-skinned individuals.10 Real-time fluorescence imaging has become an integral tool in the diagnosis and treatment of patients with chronic wounds.
During the conduct of the FLAAG trial,5 the investigators observed an unexpected clinical phenomenon in chronic wounds: bacterial fluorescence aggregated at the edge of some wounds. This was most pronounced in diabetic foot ulcers (DFUs). The senior author coined the term ‘ring of fire’ to describe this clinical observation. Representative patients taken from the FLAAG clinical trial are presented to demonstrate the ring of fire. This is the first clinical report of this phenomenon.
Methods
The FLAAG clinical trial (ClinicalTrials.gov identifier: NCT03540004) evaluated 350 patients with a variety of chronic wounds.5 The trial was a prospective, single-blind, multicenter, cross-sectional study conducted across 14 advanced outpatient wound care centers in the United States from May 2018 to April 2019. Adult patients (≥18 years) with wounds of uncertain infection status were enrolled, and the wounds were assessed and imaged by 20 clinicians with expertise in wound care, including surgeons, podiatrists, and nurse practitioners. Participants were excluded if they received an investigational drug in the prior 30 days, had undergone a wound culture within the past month, were unable to provide consent, or had wounds located in areas unsuitable for imaging.
Ethical approval for the study was obtained from an independent Institutional Review Board (Veritas IRB, Montreal, QC). All patients signed an IRB-approved consent prior to the performance of any trial related procedures. The six case studies presented here were drawn from this trial.
In the FLAAG trial, confirmation of bacterial identification by fluorescence imaging was confirmed with quantitative tissue culture biopsies taken from the center and edge of the wound. A biopsy was taken of all fluorescence-positive areas.
Fluorescence imaging utilizing the MolecuLight device (MolecuLight, Toronto, CA) illuminates the wound bed with a safe violet light at 405nm. This specific wavelength excites endogenous porphyrins in most bacteria and pyoverdines in Pseudomonads. The porphyrins emit their own red light that is detected by the device and quantified. Pyroverdines emit a cyan light. The MolecuLight device is approved by the FDA for the detection of Pseudomonas. MolecuLight will also identify the location of the bacteria in the wound bed and on the periwound. No contrast agents are required.
Case reports
Case 1
A 44-year-old male with newly diagnosed non-insulin dependent type 2 diabetes developed a neuropathic DFU located on the plantar aspect of the left foot. The wound had persisted for 2 months. The patient was not on systemic antibiotics.
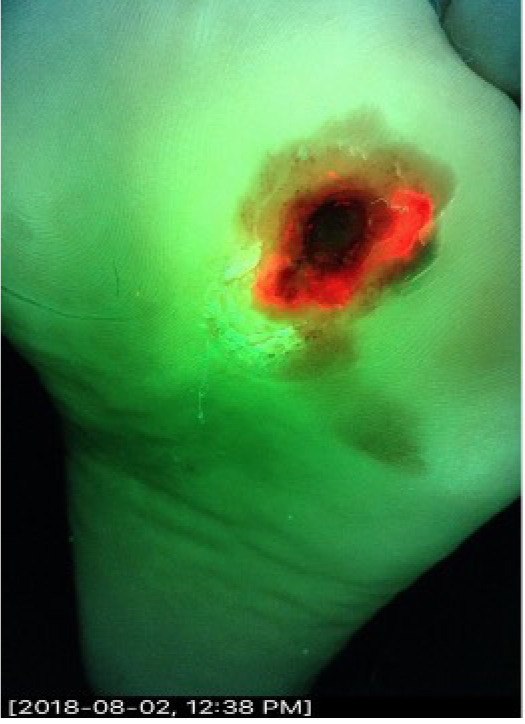
The wound measured 1cm x 0.6cm x 0.3cm. CSS assessment by the investigator was negative for bacterial burden. The fluorescence image (i:X) was positive (Figure 1). A quantitative biopsy of the center of the ulcer demonstrated light bacterial growth on quantitative tissue culture. The iX-fluorescence image-guided biopsy demonstrated heavy growth. Cultures grew Streptococcus agalactiae, Enterococcus faecalis and Finegoldia magna.
FIGURE 1 The ‘ring of fire’ in a diabetic foot ulcer.
Case 2
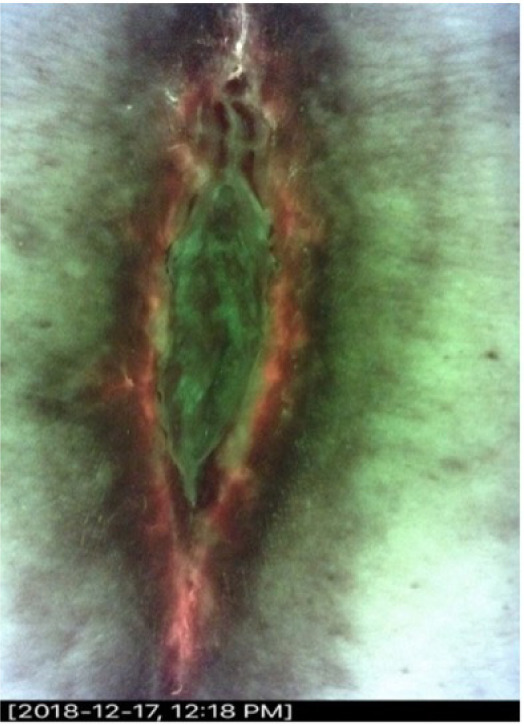
A 55-year-old female presented with a surgical site infection following lumbar back surgery. The wound became infected and dehisced 12 months prior. She was initially treated with systemic antibiotics, but they were stopped several months before the study. The wound measured 6.5cm x 1.5cm x 1.2cm. The initial CSS assessment was negative but CSS + iX was positive (Figure 2). The center of the biopsy was negative. The edge biopsy grew Corynebacterium species.
FIGURE 2 Surgical site infection following lumbar back surgery. The wound exhibits a ‘ring of fire’ pattern. This pattern demonstrates bacterial aggregation at the wound periphery.
Case 3
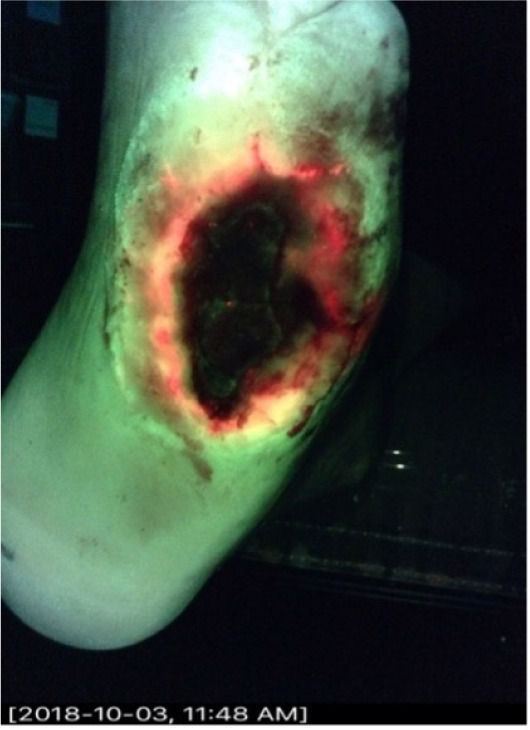
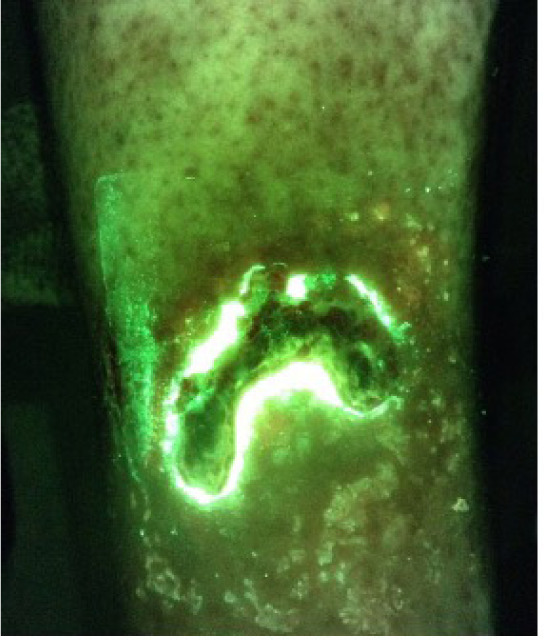
A 45-year-old diabetic male presented with a DFU of 12 months duration. Systemic antibiotics were stopped 2 weeks prior. The ulcer was worsening. The wound measured 4.5cm x 2cm x 0.3cm. The initial CSS assessment was negative, and the CSS + iX fluorescence assessment was positive (Figure 3). Quantitative biopsy from the wound margin grew Enterococcus faecalis, Staphylococcus aureus, Klebsiella oxytoca, and Serratia marcescens.
FIGURE 3 A 45-year-old diabetic male with a diabetic foot ulcer of 12 months duration.
Case 4
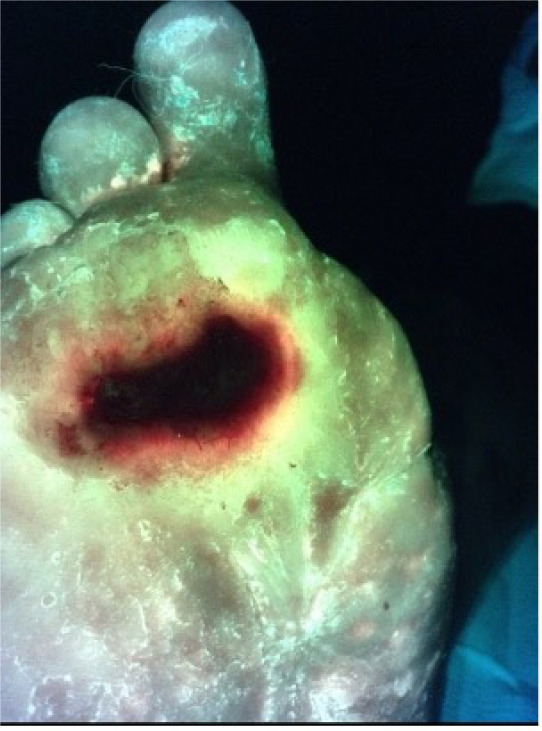
A 58-year-old male with a history of type 2 diabetes who presented with a 12-month history of a DFU that started after wearing new ill-fitted shoes. The ulcer was located on the plantar foot. The patient was not on systemic antibiotics.
The wound measured 2.4cm x 1.5cm x 0.2cm. The initial CSS assessment was negative, and the CSS + iX fluorescence assessment was positive (Figure 4). The quantitative biopsies from the wound edge grew Staphylococcus aureus and Enterococcus faecalis species.
FIGURE 4 The diabetic foot ulcer shows a ‘ring of fire’ appearance, characterized by bacteria densely concentrated around the wound margins.
Case 5
Most bacteria exhibit red fluorescence due to excitation of endogenous bacterial porphyrins by the 405nm violet light emitted by the MolecuLight imaging device; however, Pseudomonal species are an exception. Pseudomonads possess endogenous pyoverdines rather than porphyrins. As a result, they emit a cyan fluorescence wavelength of light when exposed to the 405nm excitation light from the device.11
A 65-year-old male presented with a venous leg ulcer wound located on the medical gaiter region of the ankle. The wound had been present for 12 months and periodically opened and closed during that time. It measured 3cm x 2.2cm x 0.3cm. Initial CSS assessment was negative and CSS + iX was positive (Figure 5). Quantitative biopsy of the center of the wound was negative. The quantitative biopsy from the wound margin grew heavy amounts of Pseudomonas aeruginosa accompanied by Enterococcus faecalis, Corynebacterium striatum, and Staphylococcus simulans.
FIGURE 5 The wound shows a ‘ring of fire’ pattern, where bacteria gather at the edges, a hallmark often seen in chronic venous ulcers.
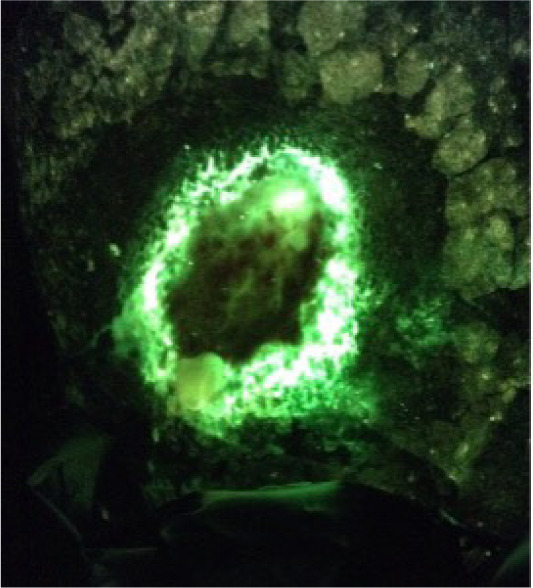
Case 6
A 68-year-old male presented with a venous leg ulcer located on his calf of 3 months duration. The patient was on long-term steroids. He was not on systemic antibiotics. The wound measured 2.7cm x 3cm x 0.2cm. The initial CSS assessment was negative. The CSS + iX fluorescence assessment was positive (Figure 6). The quantitative tissue biopsy revealed heavy growth of Pseudomonas aeruginosa.
FIGURE 6 Pseudomonal ‘ring of fire’ in a venous leg ulcer.
Discussion
This is the first publication describing the unique phenomenon seen with real-time bacterial fluorescence imaging of some nonhealing wounds – the ‘ring of fire’. The fluorescence emitted by bacterial biofilms tends to occur at the margins of some chronic wounds, where the local hypoxic tissue microenvironment is most conducive to persistent growth.10 Examination of the wound using CSS is not reliable in determining the level of bacteria in the wound and is used inconsistently in clinical practice.5 The characteristic bacterial fluorescence ring concentrated on the wound margins of some chronic wounds suggests a localized and elevated concentration of (pathogenic) bacteria, which are known to be a major barrier to effective wound healing.12
The occurrence of the ‘ring of fire’ phenomenon in nonhealing wounds is supported by the pathophysiology of chronic wound microenvironments. Chronic wounds are associated with hypoxia secondary to impaired circulation, persistent inflammation and immune system dysfunction.13 These conditions contribute to the development and persistence of bacterial biofilms. Bacterial biofilms are structured communities of bacteria encased in an extracellular matrix that protects them from these chronic wound conditions along with antimicrobial treatments. The edges of chronic wounds seen in the bacterial fluorescence ’ring of fire’ are vulnerable to biofilm formation,14 because of necrotic tissue generated by the wound healing process, reduced oxygenation and repeated mechanical stress. These conditions are the ideal niche for biofilm formation visualized by fluorescence imaging as the so called ‘ring of fire’. The biofilm clustered at the wound edge hinders wound healing by physically blocking re-epithelization and providing a reservoir for persistent infection and cross contamination between wounds in close proximity in patients.15
The ‘ring of fire’ seen in chronic wounds has implications for the practice of wound care. Eliminating bacterial bioburden is integral to promoting wound healing. In the past, practitioners have relied on CSS to identify clinically significant bacterial levels in chronic wounds; however, the FLAAG trial demonstrated that CSS is unreliable.5 Providers have also utilized semi-quantitative cultures taken from the center of the wound using the Levin technique;16 however, this technique is inaccurate and used inconsistently in the diagnosis and treatment of chronic wounds,17 and, more importantly, typically samples the center of the wound. Real-time fluorescence imaging is superior to CSS and standard cultures in detecting bacteria and directs the clinician to the wound margin in patients observed to have ‘the ring of fire’. This finding also stresses the importance of sharp debridement of the wound margin as part of the management of nonhealing wounds.
Conclusion
The bacterial fluorescence ‘ring of fire’ describes the aggregation of elevated levels of bacteria at the wound margin, visualized as a ring around the wound edge. This clinically important phenomenon is only visualized using real-time fluorescence imaging. Clinical evidence presented here highlights the importance of focusing on the wound edge when managing nonhealing wounds.