Introduction and clinical significance
Approximately 1-3 million people develop a pressure injury annually in the United States.1 These complex injuries can leave the body vulnerable to discomfort and cause prolonged complications, such as debilitating infections, sepsis, and immobility.2 Such injuries are notorious for exacerbating over time if not treated with consistent and adequate care, thereby quickly turning into chronic long-term wounds.3 Prevalence of these injuries are frequently noted in areas of the body with boney prominences that absorb pressure from prolonged immobility and an attenuation in blood flow – such as the elbow, heels, hips, sacrum, and shoulders. As such, the elderly, inpatient hospital populations, and other individuals experiencing mobility limitations are the most commonly affected by pressure injuries.1 Paralyzed patients are particularly vulnerable to pressure injuries, and the prevalence in these patients is high, with 33.9% in quadriplegics, 47.4% in paraplegics, and 9.6% in hemiplegics.4,5
These injuries vary in severity. Pressure ulcer severity is commonly classified into stages using the National Pressure Injury Advisory Panel system, which classifies ulcers as stage 1 to stage 4 or ‘unstageable’ ulcers.2,6 Stage 3 and stage 4 generally require the most intensive, costly wound management and care, as complications plus underlying patient medical history can easily lead to injuries exacerbating rapidly and potentially becoming life-threatening.2 Due to the high burden of cost, variations in healing with the use of standard care techniques, and most importantly continued difficulty in preventing pressure injuries, it is vital to evaluate new technologies to support efficacious clinical results.
In pressure ulcers where intensive wound management is necessary and the standard of care (SOC) alone has proven insufficient, use of adjunct solutions including cellular, acellular, and matrix-like products (CAMPs) has shown promise. This includes use of placental-derived allografts to support the SOC intervention by providing a natural, protective barrier from the external environment. Placental allografts vary in terms of which membrane layers are retained, leading to differences in the composition of available collagens, elastins, proteoglycans, and hyaluronic acid levels.7 As such, these natural properties have supported the use of placenta as allografts for wound coverings. This case study provides an opportunity to clinically evaluate the performance of a full-thickness amnion-chorion-amnion (ACA) allograft that features an added amnion layer, ACApatchTM (distributed by Tiger Wound Care Medical, LLC, USA). This tissue technology is a dehydrated, terminally sterilized placental derived allograft that retains the amnion layer, intermediate spongy layer, and chorion layer, plus an additional amnion layer.
In this case report, we sought to evaluate wound size and wound characteristic trends when ACA was applied over 8-weeks as an adjunct to SOC for a hard-to-heal, stage 4 pressure wound. Parameter changes were quantified and evaluated using wound surface area (SA), percent area reduction (PAR), wound volume (WV), and percent volume reduction (PVR). Additional outcomes tracked included: (1) duration of allograft applications (in days and weeks); (2) incidence of adverse events associated with use of the allograft; and (3) changes in wound characteristics.
Case presentation
The retrospective data for this case study were extracted from patient progression notes compiled during home care wound services from the single provider group (Instacare Wound Care, Illinois, USA). Data extracted include patient demographics (age, body mass index (BMI), biological sex, and ethnicity), medical history, nutritional assessment scores, pressure ulcer risk assessment scores, plus any available objective and subjective weekly measurements.
Patient presentation
This report is centered on a 71-year-old, African American, non-Hispanic, non-Latino, male patient with a BMI of 23.3. He is a former smoker, has a medical history of paraplegia, incontinence, sensory loss, malignant neoplasm of the prostate (in remission), and presented with a sacral wound categorized as a stage 4 pressure ulcer. At the time of initial encounter (prior to initial allograft application) it was noted that the patient was at an increased risk of pressure injury incidence. The Pressure Ulcer Risk Assessment (PURA), using the Braden Scale for predicting pressure sore risk, identified the patient as ‘high risk’ for developing a pressure ulcer (Braden Scale score of 11).8 Additionally, a mini nutritional assessment (MNA) was captured, with a resulting score of 10, putting the patient at high risk of malnutrition.9–12 To address the MNA grade, the patient was advised by the provider to increase his intake of protein and calories.
Wound assessments
Weekly subjective and objective wound assessments were documented at initial encounter and continued during subsequent visits by the care provider. Wound characteristics including exudate amount, exudate type, color, odor, and pain were assessed systematically and recorded per provider expertise. Linear wound size measurements (length, width, and depth) were obtained using a sterile, disposable ruler. To limit overestimation in measurements, the greatest length head-to-toe and greatest width perpendicular to the length were used. To determine depth, a sterile cotton tip swab was gently inserted into the deepest part of the wound, after which it was measured against a ruler.13
Static wound surface area (SA; cm2) measurements were calculated as length multiplied by width, whereas static wound volume (WV; cm3) was calculated as SA multiplied by wound depth. Dynamic percent area reduction (PAR; %) was calculated using the wound SA measured immediately prior to allograft application and SA measured at each subsequent application visit, such that PAR = [(SA prior to 1st allograft application – SA at subsequent applications) ÷ (SA prior to 1st allograft application) x 100]. Percent volume reduction (PVR; %) was calculated using the wound volume measured immediately prior to allograft application and volume measured at each subsequent application visit, such that PVR = [ (WV prior to 1st allograft application – WV at subsequent applications) / (volume prior to 1st allograft application) x 100].
Allograft application
Pre-allograft application checks
Prior to placental allograft application, a comprehensive clinical evaluation was conducted by the wound care provider. This included a detailed review of the patient’s medical history, health at the time of allograft application, and medical needs. The documentation confirmed that the wound had failed to demonstrate measurable improvements following more than 4 weeks of SOC management. Vascular perfusion was evaluated via ankle-brachial index tests, with resulting measurements established to be between 0.7 to 1.2. Wound bed optimization was confirmed prior to allograft application at each visit. This included ensuring appropriate moisture balance was maintained, plus wound odor, maceration, and periwound hygiene were effectively controlled. The provider also ensured infections were ruled out and the wound was deemed free of non-viable tissue, with complete removal of devitalized material.
As a commitment to patient comfort and safety, procedure preparation, risks and complications were discussed with the patient and documented accordingly. This conversation also included a review of precautionary wellbeing measures, such as adherence to tobacco cessation.
The placental allograft was selected for application at the discretion of the treating provider. Selection was based on the patient and wound presentations, including those mentioned herein. The provider’s preference for the four-layered placental allograft was also due to its multi-layered configuration, which may offer enhanced mechanical support and a more favorable wound environment per prior published literature.7
Allograft application
Following meticulous cleansing with a wound cleanser and complete removal of non-viable, devitalized tissue, the provider applied the appropriately sized allograft to the prepared wound bed, ensuring limited wastage of the product. The procedure was well tolerated by the patient, with loss of sensation noted throughout and following the procedure. The allograft was then secured by a non-adherent dressing and steristrips. For offloading, the provider recommended repositioning every 2 hours while avoiding direct pressure to the wound site. Additionally, a barrier ointment was ordered to protect the periwound, including post incontinence episodes.
After the initial allograft application, subsequent weekly follow-up visits were ordered to evaluate the wound progress and for reapplication of the ACApatchTM allograft. At each weekly visit, the provider performed a comprehensive reevaluation of the wound, cleansed the wound bed, and reapplied the allograft with appropriate primary and secondary dressings. SOC and allografts applications continued for 8 weeks. There were no reported adverse events related to the application of the allografts.
Clinical findings and outcomes
Allograft application cadence and wound attenuation trends are summarized in Table 1, which outlines the weekly evaluation intervals, placental allograft application frequency (in days and weeks), and corresponding values for the wound metrics assessed. Analysis for SA, PAR, WV, and PVR parameters demonstrated a consistent week-to-week pattern, which reflects pressure wound dimensions attenuating overtime. To further illustrate the findings, the subsequent figures in this case study complement the data in Table 1 through graphical representations of the size and volume regression trends (Figures 1-3).
TablE 1 Allograft application cadence and wound progression
| Evaluation timepoints | Measured duration in weeks | Measured duration in days | Wound care | Surface area* (cm2) | PAR (%) | Wound volume (cm3) | PVR (%) |
|---|---|---|---|---|---|---|---|
| Application intervals | Wound care plan | SA = length x width | Using SA from pre 1st ACA timepoint for PAR calculation | WV = surface area x depth | Using WV from pre 1st ACA timepoint for PVR calculation | ||
| 1-week pre initial ACA | SOC only | 215.28 | 861.12 | ||||
| Pre 1st ACA | Week 0 | Day 0 | SOC + allograft | 219 | 876 | ||
| Pre 2nd ACA | 1 week | 7 days | SOC + allograft | 200.2 | 8.58 | 700.7 | 20.01 |
| Pre 3rd ACA | 2 weeks | 14 days | SOC + allograft | 179.4 | 18.08 | 448.5 | 48.80 |
| Pre 4th ACA | 3 weeks | 21 days | SOC + allograft | 160 | 26.94 | 320 | 63.47 |
| Pre 5th ACA | 4 weeks | 28 days | SOC + allograft | 77 | 64.84 | 154 | 82.42 |
| Pre 6th ACA | 5 weeks | 35 days | SOC + allograft | 56 | 74.43 | 112 | 87.21 |
| Pre 7th ACA | 6 weeks | 42 days | SOC + allograft | 50.4 | 76.99 | 100.8 | 88.49 |
| Pre 8th | 7 weeks | 49 days | SOC + allograft† | 42 | 80.82 | 42 | 95.21 |
| 1-week post final ACA | 8 weeks | 56 days | SOC only | 36 | 83.56 | 36 | 95.89 |
*Surface Area (SA) and Wound Volume (WV) measurements obtained prior to each allograft application. †ACA application up to 8-weeks only at the discretion of the care provider. Table 1 outlines the patient’s weekly intervals, wound care paradigm, allograft application cadence, and dimension-based wound assessment metrics. Relevant assessments included wound SA (cm2) and WV (cm3), recorded weekly from 1-week pre initial allograft application to 1-week post final application; the respective PAR (%) and PVR (%) for each visit were subsequently calculated. Measurements demonstrated a consistent, weekly attenuation of wound dimensions over the course of applications.
FIGURE 1 Weekly change in wound surface area. Figure 1 depicts wound SA (cm2) at each weekly visit, from 1-week pre initial allograft application to 1-week post application. Analysis demonstrated a strong linear trend across weekly intervals, reflected by a coefficient of determination of 0.92. This progressive downward trajectory is indicative of continuous wound size reduction.
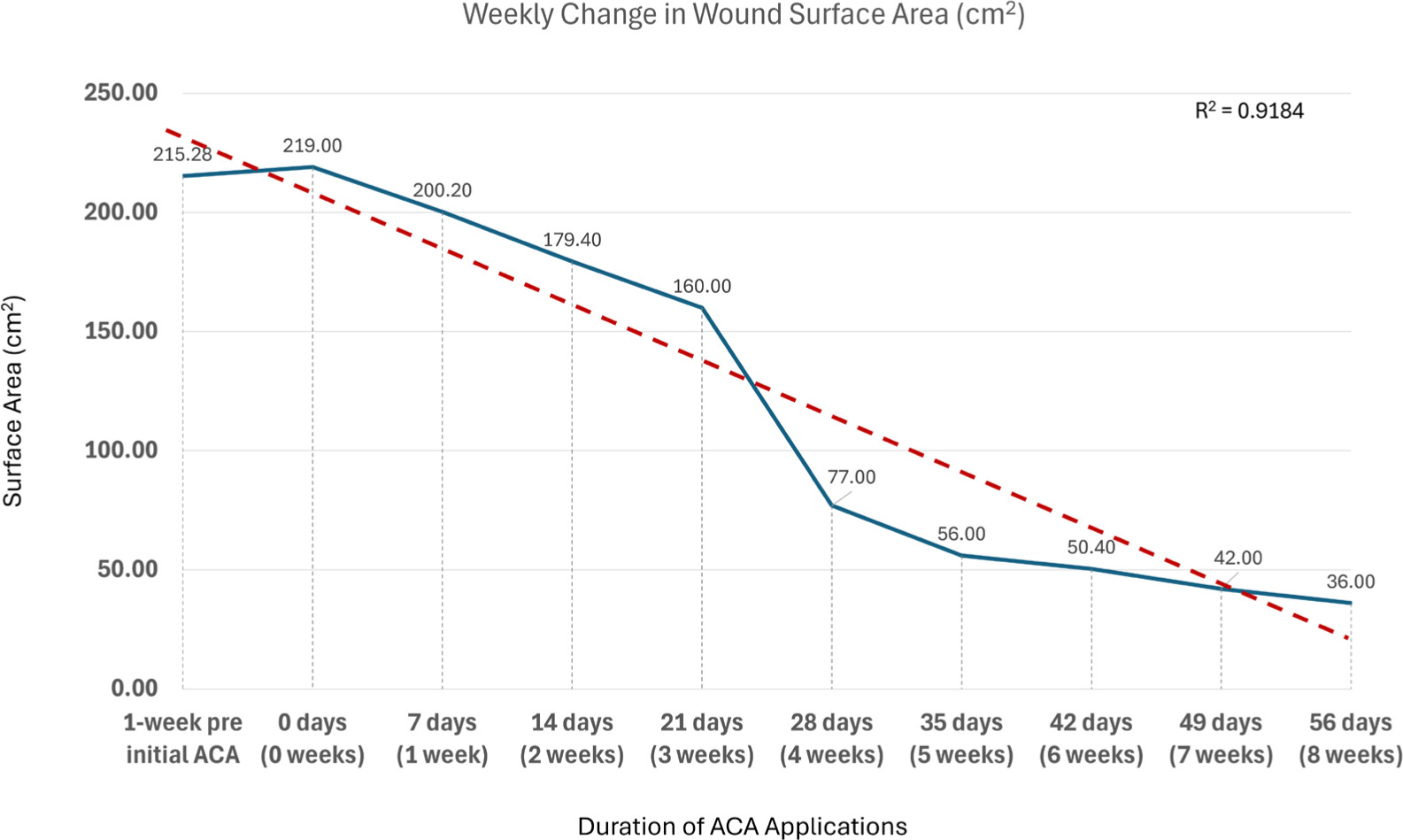
FIGURE 2 Percent area reduction per weekly evaluation timepoints. Figure 2 depicts the weekly Percent Area Reduction (%) of the pressure wound, from post-first to post-final allograft application. A strong linear trend, with a coefficient of determination of 0.88, across weekly timepoints can be observed. This upward linear trajectory reflects an increasing magnitude of percent change when the placental allograft is applied in conjunction with standard of care.
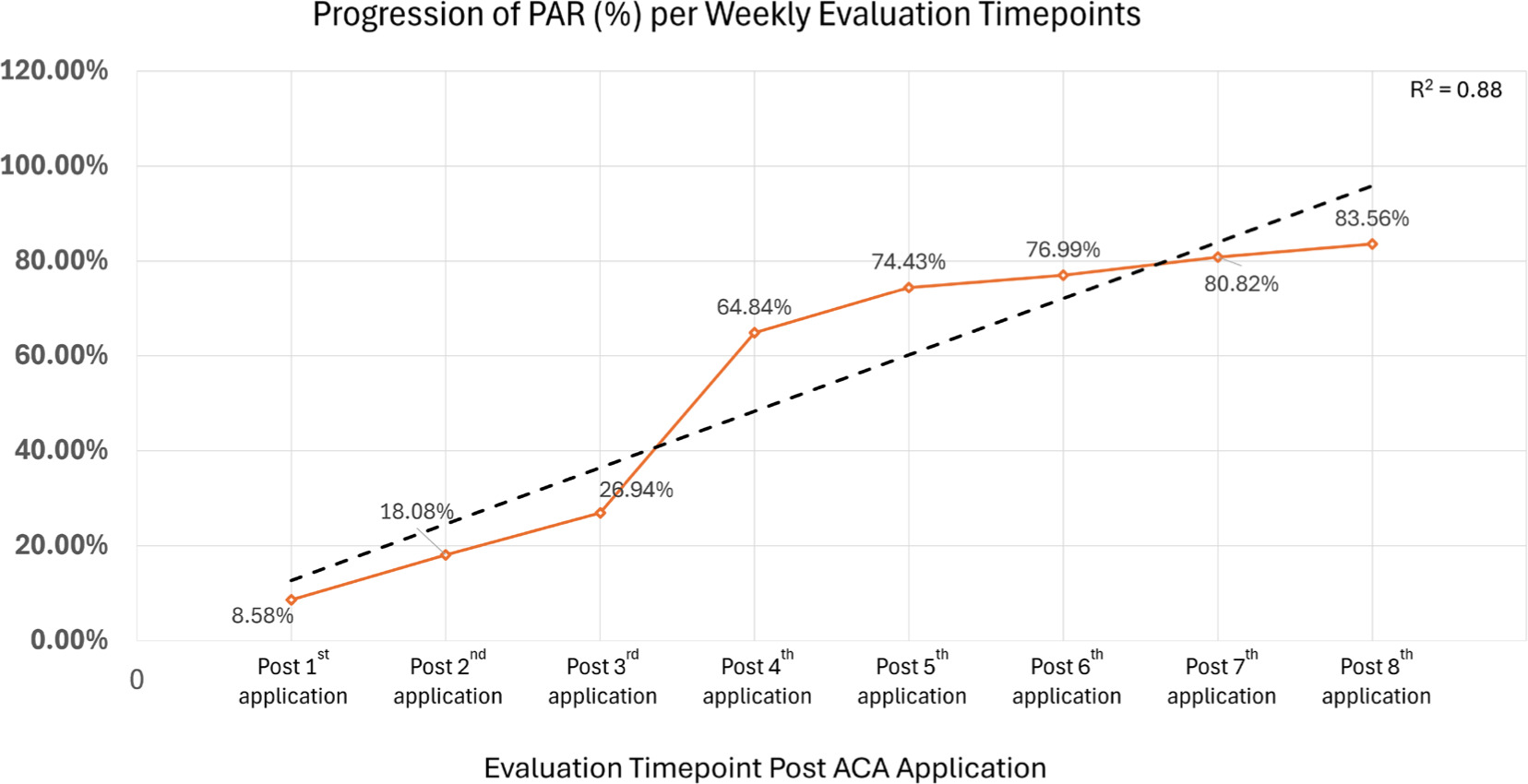
FIGURE 3 Weekly change in wound volume and percent volume reduction. Figure 3A depicts wound volume (cm3) at each weekly visit, from 1-week pre allograft application initial to 1-week post final application. A strong linear trend, with a coefficient of determination of 0.90 across weekly timepoints, can be observed. This downward progressive trajectory is indicative of continuous wound size reduction. Figure 3B depicts weekly percent volume reduction (%) values from post-first to post-final allograft application. A strong linear trend, with a coefficient of determination of 0.84, across the weekly timepoints can be observed. This upward linear trajectory reflects an increasing magnitude of percent change when the placental allograft is applied as an adjunct to standard of care.
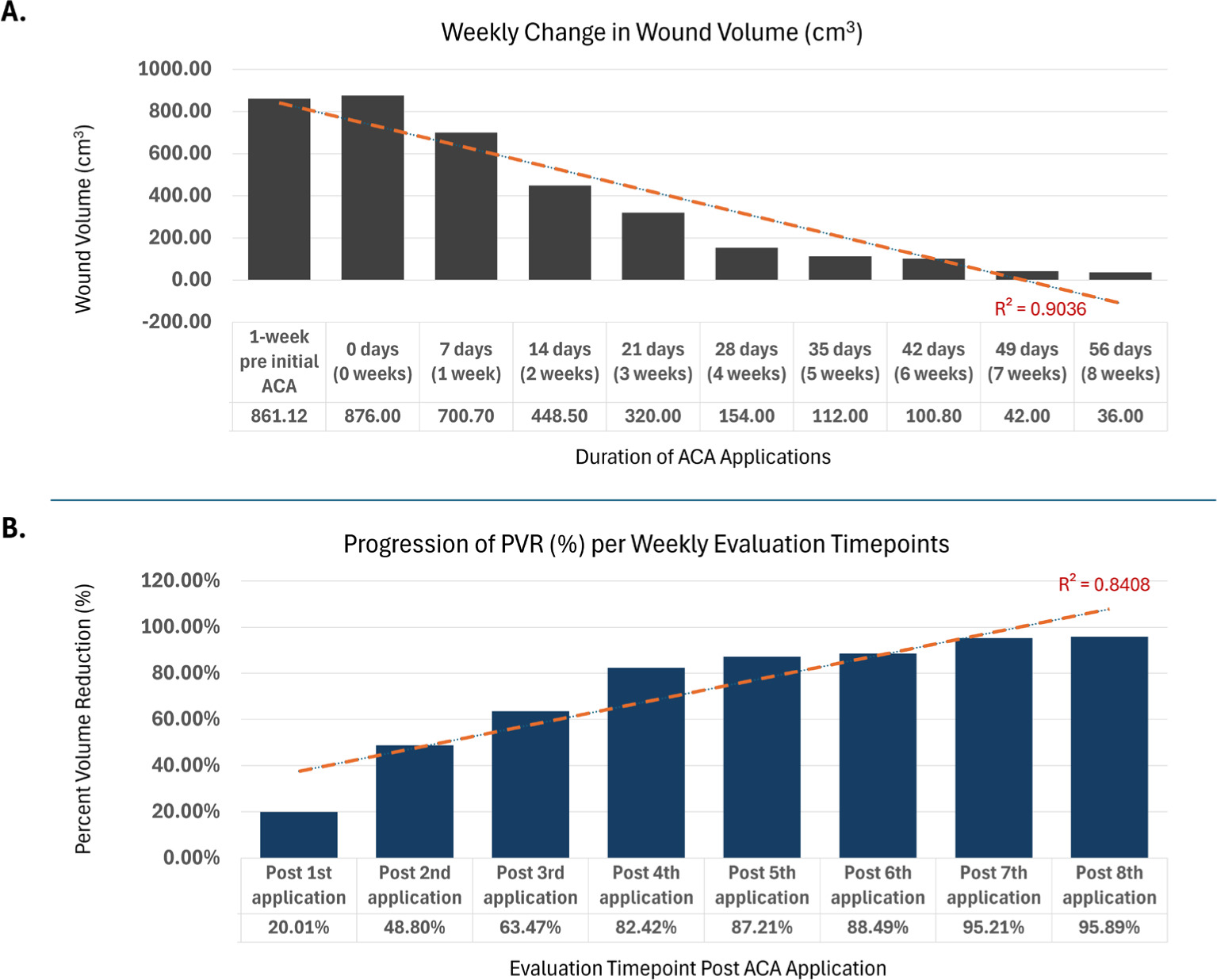
Static parameters, specifically surface area (Figure 1) and wound volume (Figure 3A), demonstrated strong linear trends across the weekly evaluation timepoints, with both exhibiting downward trajectories indicative of continuous wound size reduction. Surface area decreased from a measurement of 219cm2, captured immediately prior to initial allograft, to 36cm2, measured after 8 weeks of SOC and adjunctive allograft applications, corresponding to a 83.56% area reduction. Wound volume demonstrated a similar progressive decrease from 876cm3, captured immediately prior to initial allograft, to 36cm3, measured after 8 weeks of allograft application with SOC, corresponding to 95.89% volume reduction. The linear regression models for these measures depict high coefficients of determination (R2) of 0.92 and 0.90, respectively, underscoring the consistency and reliability of the observed trends, supporting the overall validity of the captured measurements.
Conversely, dynamic wound assessment measures, specifically percent area reduction (Figure 2) and percent volume reduction (Figure 3B) exhibited upward linear trajectories, reflecting an increasing magnitude of percent improvement as SOC and adjunctive allografts are applied at subsequent weeks. PAR increased from a measurement of 8.58%, captured post initial allograft, to 83.56%, measured after 8 weeks of SOC and adjunctive allograft applications. WV demonstrated a similar progressive increase from 20.01%, taken post initial allograft, to 95.89%, measured after 8 weeks of allograft application with SoC. Similarly to SA and WV trends, these also demonstrated high coefficients of determination, with R2 being 0.88 for PAR and 0.84 for PVR.
In summary, a mean weekly PAR of 10.4% was observed over the 8-week interval period, calculated as the final PAR (after completion of 8 applications) divided by the total number of interval weeks. In the week preceding the initial allograft application, the pressure wound demonstrated an increase in size dimensions, suggesting ongoing wound worsening even though SOC measures were actively being performed. Following the initiation of the allograft applications, consistent directional trends were observed across the area and volume metrics, reflecting progressive wound reduction in dimensions over time.
Discussion
Previous research has demonstrated that the four-layer full-thickness ACApatchTM retains higher levels of proteins, including extracellular matrix components.7 Based on these observations, such allografts may offer enhanced mechanical support and a more favorable wound environment in clinical settings.7 The case study presented here builds upon that previous research, by documenting the outcomes observed when applying ACApatchTM as an adjunct to the SOC in caring for a patient with a complex, hard-to-heal sacral wound. For this patient the selected intervention proved positive, suggesting that application of such ACA allografts alongside SOC warrants further investigation, and could hold promising potential in certain complex wound care protocols.
In this study, the patient exhibited a consistent weekly decrease in both wound surface area (R2=0.9184) and wound volume (R2=0.9036), as well as an overall PAR of 83.56% and PVR of 95.89% after the eighth, final ACA application. Importantly, this successful outcome was observed for a patient with significant comorbidities contributing to the increased complexity of a hard-to-heal sacral wound: paraplegia, incontinence, and sensory loss, as well as a high risk of malnutrition based upon the MNA score.
This study also yields further clinical evidence on the use of placental allografts for exceptionally large chronic wounds. For contrast, systematic reviews documenting the use of placental allografts in diabetic foot ulcers (DFUs) commonly report mean wound surface areas between 1–5cm2; those entries addressing larger DFUs typically report surface areas in the 8–12cm2 range.14–16 Studies assessing placental allograft use for venous leg ulcers (VLUs), while less-common, often adopt exclusion criteria that limit the size of eligible wounds and report upon VLUs averaging 6–10cm2 in surface area.17,18 A 2016 case series examining allograft use in large, complex, non-healing wounds included an 80-year-old male who presented with a non-healing stage 4 sacral pressure ulcer with underlying osteomyelitis, which developed following hospitalization and resection of a small bowel obstruction.19 However, the large wound in that case measured 2.9cm x 1.2cm x 0.5cm (area: 3.5 cm2, volume: 1.7cm3), a considerably smaller sacral pressure ulcer than the pressure ulcer cared for in this case study (219cm2 and 876cm3, respectively).19 Collectively, this prior findings provide valuable support for the application of placental allografts for complex, large chronic wounds.
Since this is a N of 1 case study, authors acknowledge there are limitations which can restrict the ability to generalize outcomes or draw comparative inferences. Additionally, in the absence of a control group, potential confounding variables cannot be fully ruled out. It is also noted that data analysis for retrospective real-world case studies is dependent on available data collected at time of prior patient encounters, not necessarily for standardized, controlled research purposes.
Conclusion
Use of the four-layer ACA placental allograft as a protective barrier alongside SOC intervention for a complex sacral wound proved promising. The allograft was well-tolerated, and the patient outcomes were noteworthy, including over 83% PAR (R2=0.88) and over 95% PVR (R2=0.8408) over the 8-week evaluation interval. Additional research is recommended to address the limitations inherent in this single case study model and to further improve clinical understanding of the wound care capabilities associated with the referenced ACA placental allograft. The preliminary evidence presented in this case report suggests there is potential to include application of four-layer placental allografts in certain complex wound care intervention protocols.