Introduction
A wound is a disruption to the integrity of the skin that leaves the body vulnerable to discomfort, infection, and further complications. When wounds do not respond to standard of care (SOC) alone, they become classified as non-healing or chronic wounds, a condition that affects more than 10 million residents in the United States, or 2.5% of the population.1 In these cases, SOC often proves insufficient, necessitating additional interventions to prevent morbidity and extended hospital stays.2
Advances in wound care have led to the development of innovative therapeutic modalities, including the use of allografts that are used in addition to standard treatment protocols. Placental allografts, which fall under the category of cellular, acellular, matrix-like products (CAMPs), have emerged as a promising option due to their rich composition of extracellular matrix (ECM) components and growth factors. These features contribute to the tissue’s use as a physical barrier to protect against external contaminants.3 The use of CAMPs in the medical field began in the early 1900s in skin transplantation applications and expanded in the 1980s to broader clinical applications in wound management, including treatment of both acute and chronic wounds.3,4,5
Traditional placental allografts have primarily focused on the amnion layer due to its rich ECM composition. However, the placenta is naturally composed of three distinct layers including the amnion, intermediate layer (IL), and chorion, each contributing unique biological components to support wound protection.6 The amnion layer provides protective covering, while the intermediate and chorion layers provide additional ECM structural components and growth factors to foster a supportive microenvironment that contributes to the durability and flexibility of the allograft.7,8
Recent allograft advancements have led to the development of full-thickness grafts which retain all three placental membrane layers. One such product is CompleteFT, a minimally manipulated full-thickness placental tissue allograft that preserves the three layers in their native configuration.9 With minimal processing, CompleteFT maintains the placenta’s rich ECM and retains naturally occurring growth factors. Moreover, CompleteFT reduces reliance on synthetic materials and leverages the intrinsic protective properties of the tissue.9 Its multi-layer design may confer additional durability, conformability, and ease of handling attributes that can be advantageous when managing complex or non-healing wounds.
This retrospective, observational case series evaluates the clinical outcomes of CompleteFT as applied to non-healing, chronic lower extremity wounds of varying etiologies. It provides insights into the potential benefits of placenta derived allografts, like CompleteFT, as valuable options for wound management.
Materials and methods
Ethical approval and patient consent
Advarra Institutional Review Board (IRB) reviewed this case series. Using the Department of Health and Human Services regulations found at 45 CFR 46.104(d)(4), the IRB determined that this series is exempt from IRB oversight. Research measures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as amended in 2013. The collection and evaluation of all protected patient health information were performed in a HIPAA-compliant manner. To protect the privacy and confidentiality of the patients, all identifiable information is anonymized. General patient consent (which encompasses the use of data and imaging) was obtained by the patient provider.
CompleteFT product meets all criteria to be compliant with Human Cells, Tissues and Cellular and Tissue-Based Products (HCT/P) that are regulated solely under section 361 of the Public Health Service Act and under 21 CFR Part 1271. Product was acquired and manufactured by RegenTX Partners, LLC, an affiliate company of Tiger BioSciences.
Patient data
Retrospective patient data for this case series were collected from a single provider at Healix Wound Care, a single mobile wound center, between December 2024 and February 2025. Records of adult patients (>18 years of age) with non-healing lower extremity ulcers who received CompleteFT allografts in the domiciliary outpatient setting via on-site evaluation and management from the wound center were included in this analysis. Patients with varying numbers of wounds and wound etiologies were included.
Chronic wounds were defined as non-healing for 30 days or more. Patients were included in this case series if they had a clinical diagnosis of one or more venous leg ulcers (VLUs), diabetic foot ulcers (DFUs), full-thickness traumatic wounds (TWs), and/or full thickness pressure injuries (PIs). Eligibility criteria required:
-
Wounds be present for greater than or equal to 30 days;
-
Demonstrate less than 50% reduction in total surface area within four weeks following standard of care;
-
Wound is not infected;
-
Wound surface area is greater than or equal to 2cm2 at initial presentation;
-
CompleteFT was applied.
Patient demographic data collected and analyzed included age, body mass index (BMI), sex, ethnicity, and co-morbidities such as any underlying diseases that could impact wound healing (i.e., diabetes, vascular disease, advanced age, limited mobility, smoking and concomitant medications). Primary measure of wound improvement was evaluated by calculating progression of wound surface area over time. A disposable paper ruler was used to manually collect the length and width of the wound. This endpoint was chosen for primary assessment as it is a reproducible and meaningful quantitative measure, which is effective in demonstrating even the most minor changes seen within evolving wound margins.10
Patient assessments
An internal clinical decision tree was utilized as part of the clinical practice to guide practitioners when screening wounds appropriate for CAMPs, which includes the Centers for Medicare and Medicaid (CMS) Local Coverage Determination (LCD) guidelines. As best practice, individuals must meet all clinical criteria outlined in the LCD and the wound must be designated as non-healing after 30 days of standard wound care.
Patient assessments were comprehensive and included a complete medical history with focused evaluation of each wound prior to initiation of wound care and allograft application. The following wound characteristics were reviewed by the practitioner at initial case presentation: wound etiology, wound duration, wound measurements, presence of tunneling or undermining, presence of anatomical structures such as bone or tendon, wound bed characteristics, condition of peri-wound, wound exudate, wound odor, and outcomes. Notations related to wound treatment encompassed wound bed preparation, debridement technique (autolytic, sharp excisional/non-excisional, enzymatic), choice of wound cleansing agent, CompleteFT application details, dressings, compression therapy, and offloading measures/interventions. It is important to note, that although not all of the aforementioned assessments were included in the analysis of this case series, they were evaluated by the practitioner at the time of each patients’ initial admission to care and appropriate wound care practices were followed throughout the patient’s care, per provider discretion.
Allograft application
CompleteFT product size was determined by the provider with a goal to cover all desired wound bed areas for application but not exceed wound edges. CompleteFT was applied according to the product’s instructions for use. Once applied, the graft was secured with steri-strips by the provider. Wound size was measured pre- and post-wound bed preparation by the provider applying CompleteFT every week (per provider discretion).
Post allograft application weekly care and dressings
Weekly re-assessment was performed on-site at Healix Wound Care and included re-assessment of the wound size and characteristics, as well as serial excisional sharp debridement in conjunction with dressing selection to facilitate autolytic wound healing.
At each assessment, previous dressings were removed and the wound bed was thoroughly cleansed before preparing the wound bed with a sharp excisional debridement to remove devitalized or necrotic tissue and biofilm, and hemostasis achieved.
Use of contact layers and secondary dressings (including compression) were determined by the provider and tailored as needed to meet the standards for appropriate wound care. The layer and dressings were adjusted week-to-week as wounds progressed from the inflammatory phase and entered proliferation, which is characterized by increased granulation tissue, reduction of wound exudate, and contraction of wound edges.
Results
Medical records for 4 patients were reviewed, which included 2 males and 2 females with age ranges from 77 to 98 years old (average age: 87 years). All patients were Caucasian, did not have diabetes, and are current non-smokers. All patients resided in a domiciliary care setting with wound care supervision. Patient population characteristics are presented in Table 1. The types of wounds evaluated were TWs (n=2), VLUs (n=5), and PIs (n=1). Wounds ranged in size from 2.25cm2 to 157.5cm2 at initial presentation. CompleteFT allograft was applied to a total of 7 wounds. The number of allograft applications and subsequent dressings used for each of the wounds are expanded upon in Table 2.
TABLE 1 Patient population characteristics
| Patient | Age | Sex | BMI | Number of TWs | Number of VLUs | Number of PIs |
|---|---|---|---|---|---|---|
| Case 1 | 90 | M | 43.4 | 0 | 3 | 0 |
| Case 2 | 83 | F | 30.0 | 0 | 0 | 1 |
| Case 3 | 98 | F | 20.2 | 1 | 0 | 0 |
| Case 4 | 77 | M | 19.8 | 1 | 2 | 0 |
BMI= body mass index; PIs= pressure injuries; TWs= traumatic wounds; VLUs= venous leg ulcers
TABLE 2 Wound location, full-thickness (FT) graft application and wound dressings used
| Patient-wound no. | Wound location | No. of CompleteFT applications | CAMP size range (cm2)* | Cleansing agent | Primary dressing used† | Secondary dressing used† |
|---|---|---|---|---|---|---|
| Case 1-W1‡ | Left anterior LE vascular | 10 | 16-57 | Soap and water | Silver alginate; calcium alginate; superabsorbent; Hydrofera Blue | Unna boot; Two layer compression wrap |
| Case 1-W2 | Left dorsal foot vascular | 4 | 10-40 | Soap and water | Superabsorbent; Hydrofera Blue | Unna boot; Two layer compression wrap |
| Case 1-W3 | Right lateral LE vascular | 2 | 16 | Soap and water | Silver alginate; calcium alginate | Unna boot |
| Case 2 | Right heel pressure injury | 4 | 6 | Normal saline | Triad paste; calcium alginate | ABD pad and fluff gauze bandage roll |
| Case 3 | Left anterior LE traumatic | 2 | 24 | Soap and water | Silver alginate; Triad paste | ABD pad and fluff gauge bandage roll; five layer silicone border foam dressing |
| Case 4-W1 | Left anterior LE traumatic | 2 | 6-18 | Normal saline | Silver alginate; calcium alginate | ABD pad and fluff gauze bandage wrap; silicone border composite dressing; Unna boot |
| Case 4-W2 | Left anterior LE proximal venous | 2 | 2-4 | Normal saline | Silver alginate; calcium alginate | Silicone border composite dressing; Unna boot; ABD pad and fluff gauze bandage roll |
*Allograft sizes are subject to change per visit, per wound. †This is a list of all dressings used to treat the wound; not necessarily used in the order shown; the type of dressing needed was assessed at each individual visit timepoint at the discretion of the physician. ‡Treatment is ongoing.
Across all patients, CompleteFT demonstrated success in supporting wound closure when used to supplement the SOC as is seen in Table 3, which depicts attenuation in wound surface area as a percent change function. Complete healing is seen for 4 of the 7 wounds evaluated, with the largest wound (440cm2) depicting an 84.4% wound surface area change by the 4-week timepoint post first CompleteFT application and the smallest wound (2cm2) depicting a 100% wound surface area change by the 2-week timepoint post first CompleteFT application. Of the wounds evaluated in this series, a less than 50% change in wound surface area was solely seen for the 2 wounds that were not treated to complete closure. This is due to one patient getting transferred to a different facility and the second patient expiring due to external factors not related to the wounds mentioned within this series. The largest wound (W1) at 440cm2 when the first CompleteFT allograft was applied is currently undergoing continued wound care. Nonetheless, at 12-weeks post initial allograft application, W1 for patient case 1 illustrates a positive healing trend with an 87.3% wound surface area change.
TABLE 3 Percent wound surface area (SA) change from initial application of CompleteFT to final/current disposition of the patient
| Patient | Wound no. |
Visit* | Wound SA (cm2) | % SA change† |
Final disposition notes | Status |
|---|---|---|---|---|---|---|
| Case 1 | W1 | 1st CompleteFT application Current disposition |
440 56‡ |
87.3% | Largest wound of the seven; healing ongoing at 12-week timepoint; positive trend is seen | Ongoing |
| Case 1 | W2 | 1st CompleteFT application Final disposition |
38.5 6 |
84.4% | Wound healed at 4-week timepoint | Healed |
| Case 1 | W3 | 1st CompleteFT application Final disposition |
17.6 0 |
100% | Wound healed at 3-week timepoint | Healed |
| Case 2 | W1 | 1st CompleteFT application Final disposition |
5 2.6 |
48% | Wound healed at 4-week timepoint | Expired |
| Case 3 | W1 | 1st CompleteFT application Final disposition |
51.75 28.9 |
44.2% | Wound attenuated by 2-week timepoint | Transferred |
| Case 4 | W1 | 1st CompleteFT application Final disposition |
12 0 |
100% | Wound healed at 2-week timepoint | Healed |
| Case 4 | W2 | 1st CompleteFT application Final disposition |
2 0 |
100% | Wound healed at 2-week timepoint | Healed; transferred |
*The “1st CompleteFT Application” surface area measurement is not always the same as the measurements from the initial presentation. †Percent surface area (SA) change= [(New SA - Old SA) / Old SA] x 100%. ‡At the discretion of the practitioner the wound is still healing and will continue to receive additional care.
There were no reports of adverse events or infections associated with CompleteFT application. At the discretion of the practitioner, attenuation in wound surface area was noted by 2-weeks post CompleteFT application for all patients. This can also be seen in Figure 1, which provides weekly progression trends showing initial disposition, when the allograft was applied, and changes seen post allograft application over time. CompleteFT application timepoint is demarcated with a black circle. For patient case 1, wound W1, the dashed line in Figure 1A represents the time points when wound width measurements were unobtainable. The linear regression lines for each of the graphs, outside of Figure 1C, depict an overall inverse relationship between wound surface area (measured as cm2) and wound progression as the weeks during which the wound is cared for extends when compared to the initial disposition of the patient. For Figure 1C, the linear regression trend line depicts a slightly positive slope, but this may be attributed to the steep incline seen in wound surface area (cm2) between the 2 to 4-week time period before a steep decline.
FIGURE 1 *Figure 1A – the dashed line in Figure 1A represents the time points when wound width measurements were unobtainable. †Figure 1B, 1C, 1F and 1G - depict wounds demarked as “healed”, meaning the practitioner has deemed the wounds have healed compared to the initial progression and based on the wound characteristics. Note that in Figure 1A, the uptake seen from 157 to 480cm2 is prior to allograft placement and is not due to any infection to the wound area.
Patient cases
Patient presentation case 1
Initial report: A 90-year-old Caucasian male with past medical history significant for arthritis, peripheral vascular disease, lymphedema to the bilateral lower extremities, gout, hypertension, osteoarthritis, hyperlipidemia, diverticulosis, phimosis, lymphedema, weakness, peripheral venous insufficiency, morbid obesity, old myocardial infarction, history of nicotine dependance, cervical spinal stenosis, and duodenal ulcer with no history of diabetes. Wound specialist referral from skilled home healthcare agency to mobile wound center was made in December 2024. Hospital records note bilateral lower extremity chronic ulcers were present as far back as September 2022.
Care was provided for 3 vascular wounds, located on the right lower extremity, left dorsal foot, and left lower extremity. Upon initial examination, 2 full-thickness VLUs were scattered to the bilateral lower extremities with hemosiderin staining, diminished dorsalis pedis and tibial pulses, and sluggish capillary refill with pitting edema extending from the feet to knee. Wound beds were primarily granulated with necrotic tissue present. Wound exudate was moderate to large without exhibiting any foul odor or sign of infection. Arterial brachial index (ABI) testing was completed revealing the right popliteal artery was non-compressible. Right ankle-brachial index 1.31. The entire left lower extremity was non-compressible and ABIs unable to be determined with triphasic waveforms bilaterally.
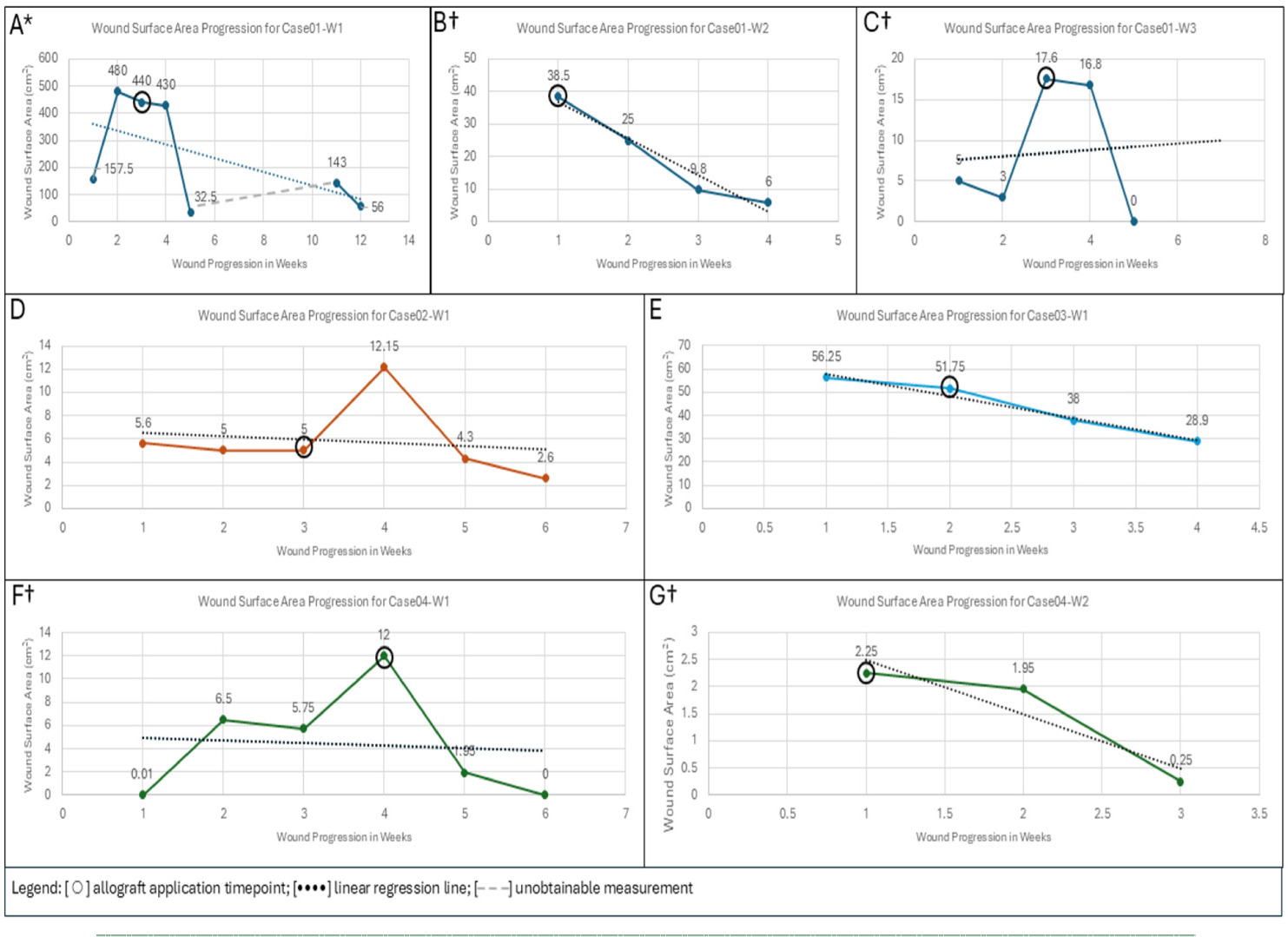
SOC including serial excisional and autolytic debridement had been performed for these wounds and was not successful. The patient provided history of vascular intervention in addition to outpatient wound center evaluation and treatment. Post application of CompleteFT, there were no reports of adverse events or infections for the three vascular wounds (Figure 2).
FIGURE 2 Case 1 – Left anterior LE vascular wound progression from initial disposition, 5-week timepoint, and current disposition after 10 applications of the allograft. Not all images for this patient are portrayed.
Patient presentation case 2
Initial report: an 83-year-old Caucasian female patient with past medical history significant for Alzheimer’s disease, hyperlipidemia, hypertension, obstructive sleep apnea, hyperparathyroidism, and gastroesophageal reflux disease with no history of diabetes or tobacco use. Wound specialist referral from skilled home healthcare agency to mobile wound center was made in December 2024 for recommendation regarding daily dressing changes presently in use to an unstageable pressure injury to the right heel. Review of medical records suggest the wound had been present since August 2024 when hospitalized.
Upon initial examination, a large eschar was centrally adhered to the right heel with peripheral edges lifting and a moderate amount of serosanguinous drainage. Dorsalis pedis and posterior tibial pulses were strong with moderate pitting edema present bilaterally from the ankle to knee. Offloading heel boots were present during initial evaluation at the bedside.
Standard of care including autolytic and enzymatic debridement had been performed for this wound and failed. Post application of CompleteFT, there were no reports of adverse events or infections for the single pressure injury.
Images for this patient are not available for use. Patient has expired with cause of death unrelated to graft application.
Patient presentation case 3
Initial report: a 77-year-old Caucasian male patient with past medical history significant for dementia with behavioral disturbance, hiatal hernia, supraventricular tachycardia, history of tobacco use, unsteady gait, frequent falls, and unintentional weight loss with no history of diabetes. Wound specialist referral from skilled home healthcare agency to mobile wound center was made in December 2024.
Upon initial examination, a large, full-thickness avulsion to the left anterior lower extremity was evaluated. Interrupted sutures were present, and the wound had dehisced with presence of necrotic tissue and a large amount of wound exudate in addition to peri-wound erythema. Dorsalis pedis and posterior tibial pulses were strong with mild pitting edema present bilaterally from the ankle to knee.
SOC including serial debridement had been performed for the wound and failed. Post application of CompleteFT, there were no reports of adverse events or infections for the single traumatic wound. Images for this patient are not available for use.
Patient presentation case 4
Initial report: A 98-year-old Caucasian female with past medical history significant for paroxysmal atrial fibrillation, chronic diastolic heart failure, moderate mitral regurgitation, sick sinus syndrome, history of left breast cancer, recurrent falls, hypothyroidism, hypercholesterolemia, gastric diverticulum, and hypertension with no history of diabetes or tobacco abuse. Wound specialist referral from skilled home healthcare agency to mobile wound center was made in January 2025.
Upon initial examination, the patient had a total of two wounds to her bilateral lower extremities, a left anterior traumatic wound and left anterior proximal venous wound. Although mild, the wounds had heavy drainage and +2-3 pitting edema was present with fragile capillaries and spontaneous purpura noted. Weeping was present upon examination with no obvious sign of infection noted. A venous ultrasound was ordered and was negative for deep venous thrombosis in either leg. ABI was then performed at the bedside by the mobile wound center and revealed left arterial brachial index of 0.88 and right ABI of 0.97, within normal limits. Medical record review revealed loop diuretic dose increase by the patient’s primary care physician during treatment.
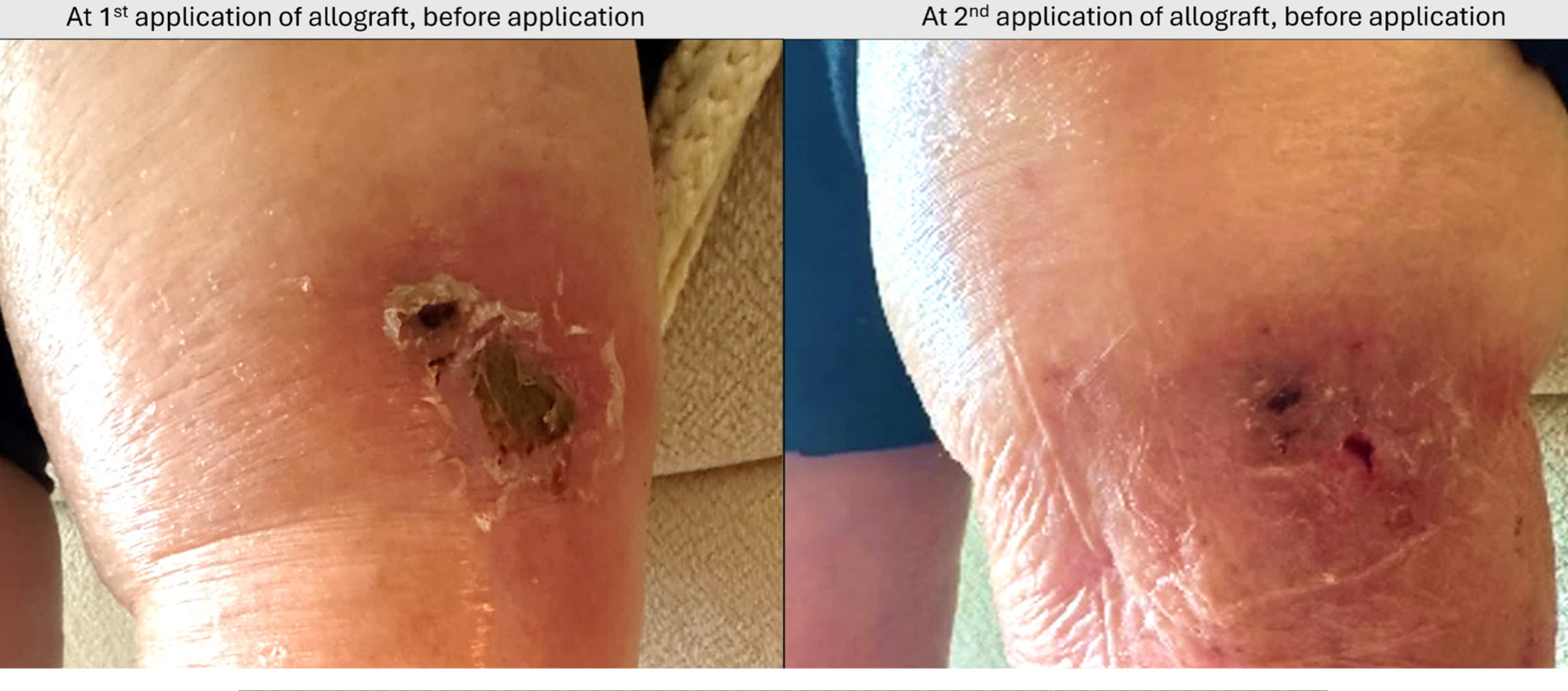
SOC including serial autolytic debridement had been performed for these wounds and failed. Post application of CompleteFT, there were no reports of adverse events or infections for the two bilateral wounds (Figure 3).
FIGURE 3 Case 4 – Left afcnterior LE proximal venous wound progression at first application of CompleteFT to second application of the allograft 1-week later. Not all images for this patient are portrayed.
Discussion
This observational case series suggests that when used to supplement SOC, CompleteFT may support successful wound closure outcomes based on empirical data. Notably, within 2 to 4 weeks of application, patients exhibited measurable wound closure progression. This rapid response highlights the potential of placental-derived materials to create a favorable wound environment when used in addition to SOC.
These promising results may be attributed to the unique structural and biological properties of CompleteFT. Unlike conventional placental allografts, which are composed of a single or partial layer of placenta membrane, CompleteFT retains all three native layers which consist of amnion, intermediate layer, and chorion.9 This comprehensive composition provides a structurally dense ECM rich with key proteins, cytokines and growth factors. Such components not only support adhesion, migration, and epithelialization but also help maintain a stable wound interface, while the immunologically privileged nature of placental allografts supports the safety profile, offering an advantage over traditional wound care material.11
The case series encompassed a diverse range of lower extremity wounds, including pressure injuries, traumatic wounds, and vascular ulcers, with sizes varying from 2 to 440cm2. Importantly, all wounds had been managed with SOC prior to the application of CompleteFT, with minimal progress in wound closure observed during that period. However, following the addition of CompleteFT to the wound care regimen, consistent wound closure was noted across patients. This finding emphasizes the challenges of healing chronic wounds in the post-acute setting, where factors such as vascular compromise, reduced mobility, and lower extremity edema act as additional barriers to sustained wound closure. Simply meeting the traditional SOC for wound healing via serial debridements and dressing selection is not enough to overcome bioburden and intrinsic healing barriers that often occur in both non-healing and lower extremity wounds, leading to adverse patient outcomes, infection, sepsis, amputation, and even death.
Limitations
It is important to acknowledge the limitations of this case series, including its small sample size and retrospective design, which limit the inclusion of control groups and the potential to compare differences across various study arms. Nonetheless, the results of the case series provide valuable preliminary insights and trends which can guide future prospective research. For example, findings from this paper can help shape the direction and focus of randomized control trials that aim to provide a robust understanding of treatment-based outcomes while minimizing confounding variables. Further research is recommended to support the case series findings and document the incremental wound healing effects when using full-thickness grafts, such as CompleteFT, in wound care management.
Conclusion
The multi-layered structure of CompleteFT, including the amnion, chorion, and intermediate layer, may contribute to its effectiveness in aiding wound healing when used alongside SOC. By preserving the natural components of the placental membrane through minimal manipulation, CompleteFT presents a valuable adjunct to address diverse wound types, including those recalcitrant to conventional treatments.