Introduction
Peripheral neuropathy, ischemia, and recurrent bacterial load disrupt wound-healing biology in diabetes and chronic venous disease, which results in persistent diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs).1-3 DFUs and VLUs drive pain, infection, limb loss, and health-system use, which results in substantial morbidity and cost burdens in older adults. Within Medicare, chronic wounds affect millions and concentrate spending in outpatient settings, which results in total annual expenditures estimated in 2018 between $28.1 and $96.8 billion.4 These epidemiologic and economic indicators underscore the need to establish DFUs and VLUs as priority indications for therapies that deliver faster closure and fewer complications.
Standard of care (SOC) protocols include debridement, reduction of bacterial burden, and proper moisture balance as well as application of off-loading to reduce plantar stress in DFUs and compression to correct venous hypertension in VLUs, which results in demonstrable gains in healing trajectory when adherence is high.5 Evidence-based guidelines from the International Working Group on the Diabetic Foot (IWGDF) endorse non-removable knee-high devices as first-line off-loading for plantar DFUs, providing superior closure compared with removable alternatives.6,7 Contemporary venous guidelines prioritize therapeutic compression as foundational therapy, which offers improved time-to-healing and reduced recurrence in VLU cohorts.3 These guideline-anchored measures define the baseline against which adjunctive cellular and tissue-based products (CTPs) must demonstrate incremental benefit.8,9
Systematic reviews of DFU treatment that aggregate the findings of randomized controlled trials (RCTs) and meta-analyses have demonstrated consistent superiority of skin substitutes over SOC with respect to complete wound closure.10 In a Cochrane Review that pooled the results across 13 RCTs, adjunctive skin substitutes demonstrated a consistently higher frequency of closure for DFUs at 6–16 weeks compared to SOC (risk ratio (RR) 1.55; 95% CI 1.30–1.85).9 Another meta-analysis of the efficacy of amniotic tissue for DFUs found a pooled healing effect of RR~2.73 (95% CI 2.06–3.61, P<0.001) favoring skin substitutes over SOC.11 Similarly, treatment of chronic VLUs has shown enhanced wound healing when skin substitutes are used adjunctively with SOC.3
Against this backdrop, through their Medicare Administrative Contractors (MACs), the Centers for Medicare & Medicaid Services (CMS) have issued Local Coverage Determinations (L39764, L39760, L35041, L39806, L39828, L39865, L39756, L36377) to create policies consistent with current evidence that covers skin substitute grafts/CTPs for the treatment of DFUs and VLUs in the Medicare population. Coverage will be provided for skin substitute grafts/ CTPs that have peer-reviewed, published evidence supporting their use as an adjunctive treatment for chronic ulcers shown to have failed established SOC methods of healing. The future effective coverage list includes 13 products indicated for treatment of DFUs and 5 products indicated for VLUs.
Within this policy architecture, the current real world evidence (RWE) study evaluated the comparative effectiveness of 2 specific Cygnus skin-substitute products using a Medicare fee-for-service claims design, which results in comparative effectiveness estimates against products listed as covered for DFU and/or VLU in the LCDs.11 This claims-based approach provides large, representative cohorts under real-world practice conditions, ensuring external validity for MAC decision-making and payer adoption. This manuscript therefore investigates outcomes for Medicare beneficiaries receiving Cygnus products versus covered comparators under SOC to assess whether Cygnus products are associated with similar or better clinically meaningful outcomes.
Materials and methods
The primary objective of this study was to determine the comparative effectiveness of 2 amnion-based Cygnus wound care products (Q4199 Cygnus Matrix, and Q4282 Cygnus Dual, Vivex Biologics, US) versus comparator CTPs accepted for coverage under the LCDs. This study used de-identified administrative claims from the 100% Medicare Fee-for-Service (FFS) Research Identifiable Files which results in complete capture of Parts A/B encounters and Part D Prescription Drug Event data for enrolled beneficiaries. CMS dissemination safeguards were applied, including the ≤20% cohort limit per year and suppression of any cell with n<11. Access, processing, and analysis of data were undertaken by an independent data management and health econometric firm (Inovalon, US).
Study design
The study employed a retrospective, observational, within-subjects design spanning January 1, 2021 through December 31, 2024, which resulted in an index-anchored episode drawn from routine care. The index date is defined as the earliest claim for a select procedure code used to treat DFUs or VLUs. Demographic measures were assessed on the index date and baseline clinical characteristics were captured in a pre-index window. Eligibility required ≥18 years of age, ≥6 months of continuous pre-index enrollment, and ≥3 months of observable follow-up. Follow-up for primary outcomes was 12 weeks post-index, providing a fixed a priori-defined observation horizon.
The index dates were not temporally aligned between the Cygnus and comparator cohorts. Each matched pair was aligned on demographic characteristics, comorbidities (CCI), and wound etiology and depth, with a uniform 6-month baseline period and 3-month follow-up period applied to all patients. Matching on index date was not feasible due to sample size constraints, but temporal overlap in data availability was maintained to ensure comparability of treatment periods.
TABLE 1 Covered Product Codes and Descriptors with Q4199/Q4282 Cohort Counts and Percentages
| Covered Product Codes and Descriptors | Q4199 Cygnus Matrix | Q4282 Cygnus Dual | ||||||
|---|---|---|---|---|---|---|---|---|
| DFU | VLU | DFU | VLU | |||||
|
(N=56) N |
% |
(N=60) N |
% |
(N=709) N |
% |
(N=334) N |
% | |
| A2019, Kerecis | 29 | 51.8% | 14 | 23.3% | 108 | 15.2% | 59 | 17.7% |
| Q4101, Apligraf | 53 | 94.6% | 56 | 93.3% | 646 | 91.1% | 306 | 91.6% |
| Q4102, Oasis | 41 | 73.2% | 46 | 76.7% | 489 | 69.0% | 238 | 71.3% |
| Q4105, Integra | 26 | 46.4% | N<11 | N/A | 140 | 19.7% | 22 | 6.6% |
| Q4106, Dermagraft | 41 | 73.2% | N<11 | N/A | 495 | 69.8% | 36 | 10.8% |
| Q4107, Graftjacket | 19 | 33.9% | N<11 | N/A | 83 | 11.7% | N<11 | N/A |
| Q4110, Primatrix | 45 | 80.4% | 40 | 66.7% | 477 | 67.3% | 189 | 56.6% |
| Q4121, Theraskin | 49 | 87.5% | 50 | 83.3% | 608 | 85.8% | 283 | 84.7% |
| Q4122, Dermacell | 20 | 35.7% | N<11 | N/A | 87 | 12.3% | 15 | 4.5% |
| Q4128, FlexHD | 29 | 51.8% | 23 | 38.3% | 164 | 23.1% | 79 | 23.7% |
| Q4133, Grafix | 55 | 98.2% | 56 | 93.3% | 684 | 96.5% | 306 | 91.6% |
| Q4151, Amnioband | 41 | 73.2% | 34 | 56.7% | 376 | 53.0% | 160 | 47.9% |
| Q4158, Kerecis Omega3 | 55 | 98.2% | 53 | 88.3% | 644 | 90.8% | 286 | 85.6% |
| Q4159, Affinity | 51 | 91.1% | 53 | 88.3% | 661 | 93.2% | 291 | 87.1% |
| Q4160, Nushield | 51 | 91.1% | 54 | 90.0% | 628 | 88.6% | 299 | 89.5% |
| Q4186, Epifix | 55 | 98.2% | 59 | 98.3% | 692 | 97.6% | 326 | 97.6% |
| Q4187, Epicord | 47 | 83.9% | 45 | 75.0% | 581 | 81.9% | 255 | 76.3% |
| Q4203, Dermagide | N<11 | N/A | 0 | 0.0% | N<11 | N/A | 0 | 0.0% |
DFU: Diabetic foot ulcer, VLU: Venous leg ulcer
In this study, propensity score modeling was not employed. Instead, a direct 1:1 matching approach was used based on predefined baseline covariates to ensure comparability between treatment groups. As this method did not involve a propensity score framework, standardized mean differences or other propensity-based balance diagnostics were not applicable.
Coding system
Cohorts were constructed using the Healthcare Common Procedure Coding System (HCPCS) on the index claim. Cygnus products serve as reference cohorts while comparator cohorts comprised covered skin substitutes frequently used for DFU/VLU care. The comparator codes are shown in Table 1.
Baseline measures
Baseline measures included age, sex, race/ethnicity, geography (region/state where feasible), dual eligibility, low-income subsidy status, and comorbidity burden by Deyo-Charlson Comorbidity Index with component conditions, providing a multidomain risk profile. Comorbidities of interest relevant to wound severity were captured using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes. These measures were drawn from the pre-index window and summarized at index.
Primary outcomes
Primary outcomes were evaluated during the 12-week follow-up with place of service (office/ASC versus. hospital outpatient) tabulated for each product. Outcomes related to comparative product effectiveness included total units billed for the index procedure; inpatient admissions and length-of-stay; emergency department visits; and lower-extremity amputations categorized as toe (minor) or foot (major). A claims-based surrogate measure of wound healing was specified as the frequency of patients who show ≥1 subsequent claim for the index procedure which includes a lower number of units than billed on the index claim, providing an objective measure of clinical response.
Detailed wound characteristics such as size, granulation tissue, and reepithelialization are not available in Medicare claims data. Instead, we used utilization-based measures, like the number of grafts applied, amputations, and care transitions, as practical indicators of wound progression within the limits of the dataset.
Duration
Therapy duration was measured over all available follow-up beyond 12 weeks. Duration was defined as time from index to the first 60-day gap without an index-procedure claim, with censoring at the end of observable data.
Direct matching
Direct matching was performed before outcome comparisons. Cygnus cases were matched to each control product in a many-to-1 fashion on age, sex, race/ethnicity, baseline Charlson score, and other clinically relevant factors. If multiple controls matched a case, one control was randomly selected to achieve a final 1-to-1 set to provide balanced matched pairs. Matching variables were drawn exclusively from the pre-index period. Following further matching on site of service (office or outpatient), Cygnus cases were compared to all covered products combined.
Statistical analysis
Statistical analyses summarize continuous variables with means/medians/SDs and categorical variables with counts/proportions. Comparative testing employed analysis of variance (ANOVA) with Tukey’s post-hoc for continuous outcomes and chi-square tests for categorical outcomes; therapy duration was analyzed with Kaplan–Meier methods and log-rank tests. Two-sided α=0.05 defined statistical significance for all comparisons. For each Cygnus product for DFUs and VLUs separately, we tabulated clinical outcome frequencies versus each covered product based on 4 criteria: Cygnus significantly (P<0.05) better than comparator (i.e., "superior"), Cygnus better than comparator but not significantly (P≥0.05), Cygnus worse than comparator but not significantly (P≥0.5), and Cygnus significantly (P<0.05) worse than comparator. Due to low frequencies, some non-significant comparisons resulted in “ties” between products. We displayed these comparisons graphically using a heat map schema (green, yellow, red) corresponding to the level and direction of statistical significance.
For comparisons that were not statistically significantly different, we tested whether non-inferiority was achieved using a standard 10% non-inferiority margin.12,13 Non-inferiority was concluded when the entire 95% confidence interval (CI) for (test – comparator) was within the pre-specified noninferiority margin (i.e., the test is not worse than comparator by more than that margin). Specifically, for binary endpoints (e.g., ≥ 1 subsequent claim, minor amputations) an absolute 10-percentage-point margin was used, testing the difference in proportions (test – comparator) with a 2-sided 95% CI. For continuous endpoints (e.g., total units billed per claim) a 10% relative margin was used (test – comparator ≤ 10% of the comparator mean). In this case, a 95% CI for the mean difference (Welch approximation) was used to determine the upper bound against the +10% margin. In comparing Kaplan-Meier medians for non-inferiority, (i.e., estimated median duration), a point-estimate noninferiority check only was applied (test median ≤ comparator median × 1.10).
Results
Eligibility criteria
Applying the eligibility criteria to the Cygnus products and covered product codes resulted in a total sample size of N=38,825 DFUs and N=23,618 VLUs. Of these, Cygnus Matrix had N=56 (0.14%) and N=60 (0.25%) and Cygnus Dual had N=709 (1.83%) and N=334 (1.41%) for DFU and VLU, respectively, available for 1-to-1 matching with each covered product. Table 1 provides frequency and percentage distributions for each covered product following the application of eligibility criteria.
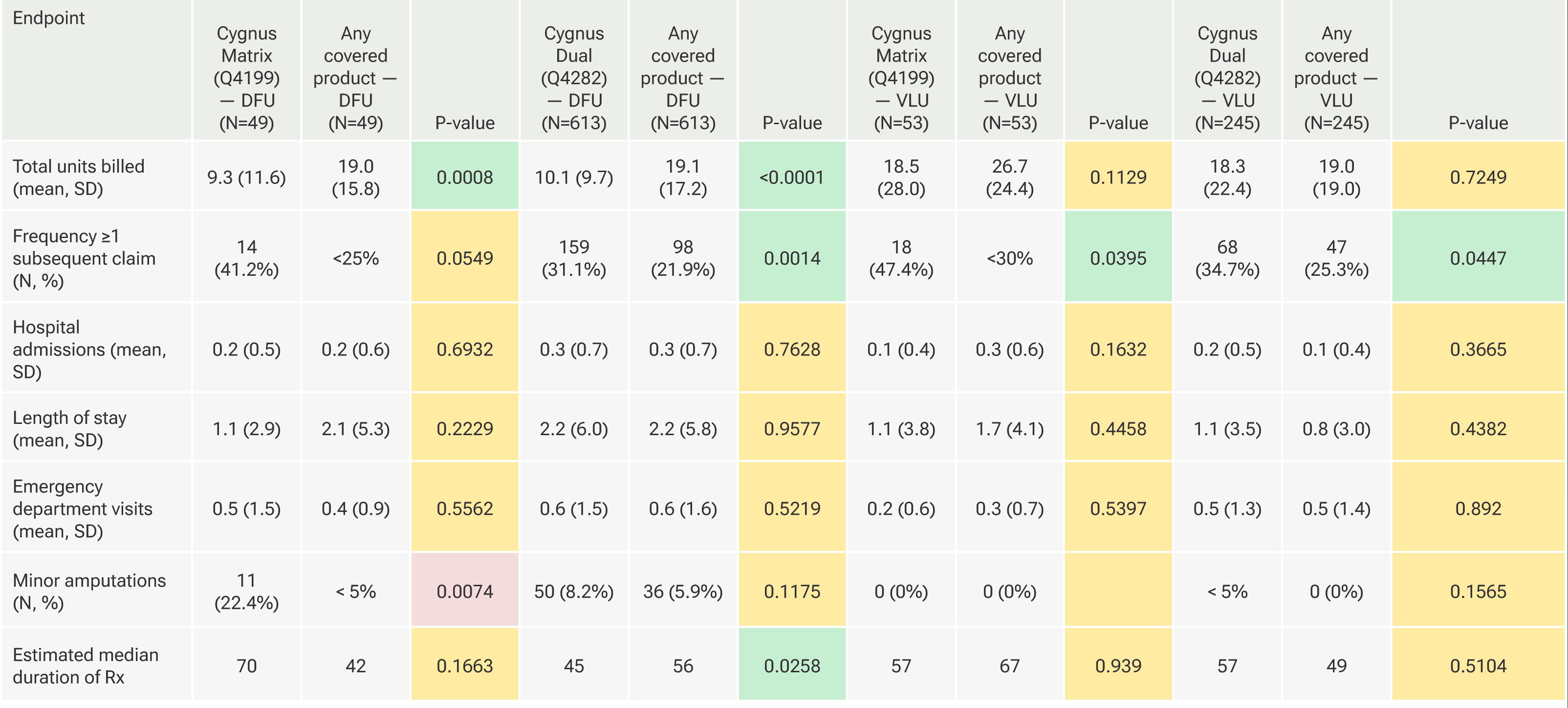
FIGURE 1 Comparisons between Cygnus Matrix and matched covered products used for DFUs.
Matching
Matching was successful in balancing background characteristics between groups with few instances of statistically significant differences. For example, there were no statistically significant baseline differences between either Cygnus product and any comparator for age, sex, race/ethnicity, low-income subsidy status, and comorbidity burden by DeyoCharlson Comorbidity Index. Almost uniformly, Cygnus products were applied therapeutically in the physician office or ambulatory surgical center, whereas place of service for comparator products most often occurred in the hospital outpatient setting.
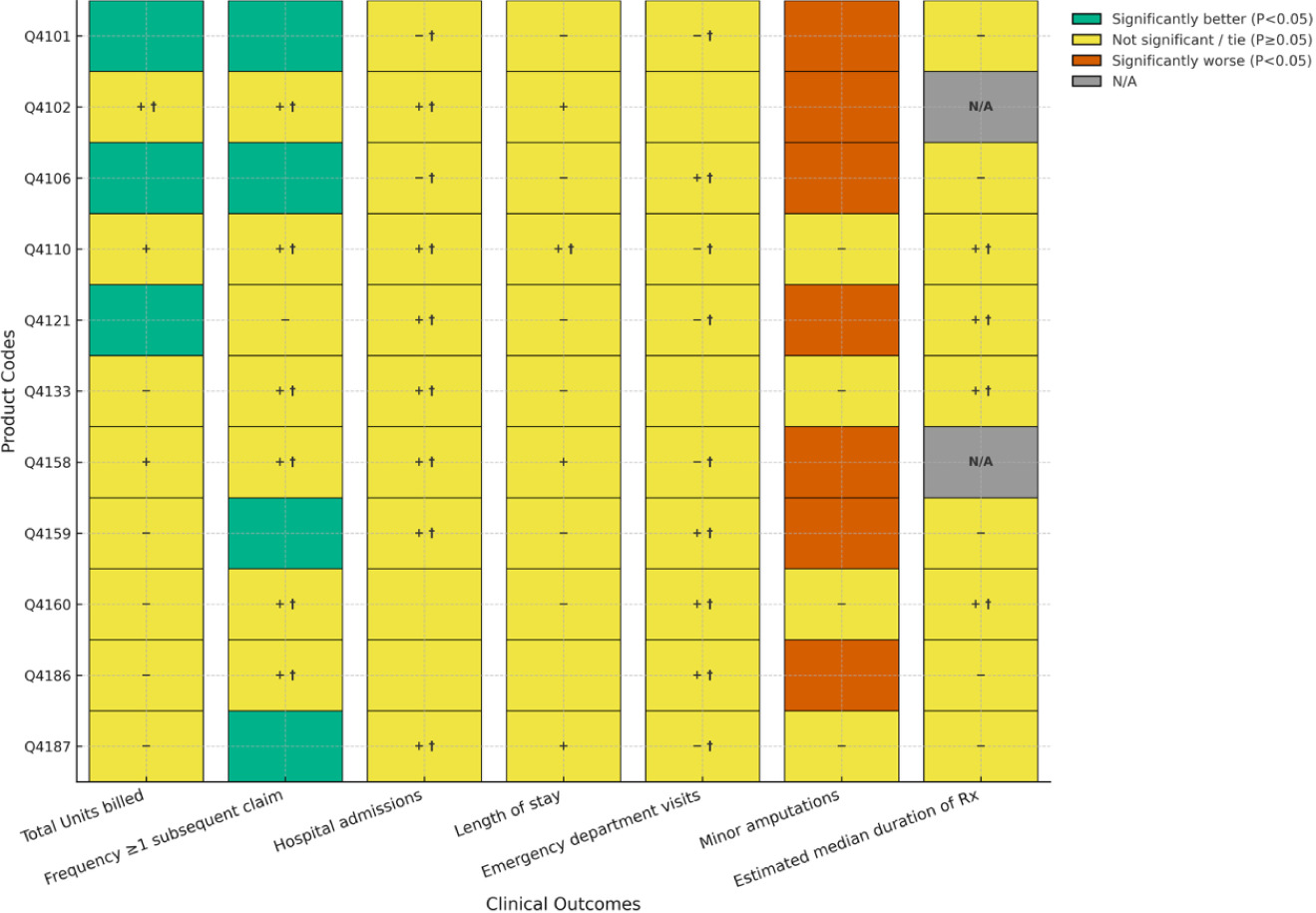
FIGURE 2 Comparisons between Cygnus Dual and matched covered products used for DFUs.
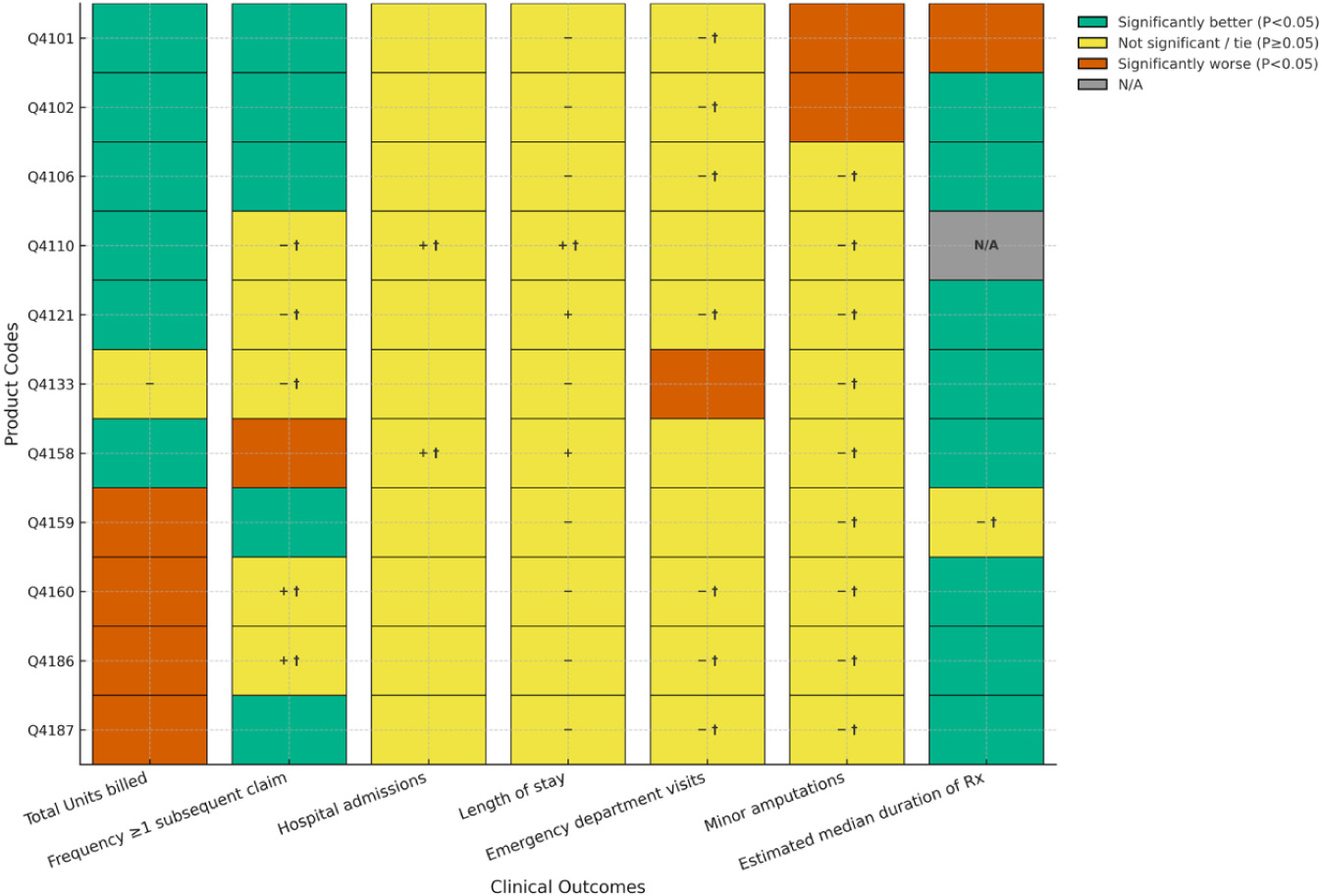
Figure 1 illustrates comparisons between Cygnus Matrix and matched covered products used for DFUs. Use of the Cygnus Matrix product for DFUs resulted in significantly fewer total units billed per claim than 27% (3 of 11) of the covered products. None of the remaining products (73%, 8 of 11) were significantly different with one comparison achieving non-inferiority. No covered product was significantly better than Cygnus Matrix on this outcome. For frequency of patients with ≥1 subsequent claim with a lower number of units billed, a surrogate measure of healing, 36% (4 of 11) of comparisons favored Cygnus Matrix over the comparator. The remaining comparisons (64%, 7 of 11) did not achieve statistical significance, with 6 achieving non-inferiority. Thus, the clinical performance with respect to healing for Cygnus Matrix was similar or better than all products on the covered list. For hospitalizations, length of stay, and emergency department visits, there were no statistically significant differences between groups for all comparisons. Non-inferiority was achieved in all (82%, 9 of 11) comparisons for hospital admissions and emergency department visits (with 2 ties each), and in a single comparison (9%, 1 of 11) for length of stay (with 1 tie). For minor amputations, 7 (64%) comparator products had significantly fewer amputations on average than Cygnus Matrix. In 4 (36%) comparisons there were no statistically significant differences with none achieving noninferiority. Lastly, there were no significant differences noted in the median duration of therapy between Cygnus Matrix and any comparator with 44% (4 of 9) achieving non-inferiority.
FIGURE 3 Comparisons between Cygnus Matrix and matched covered products used for VLUs.
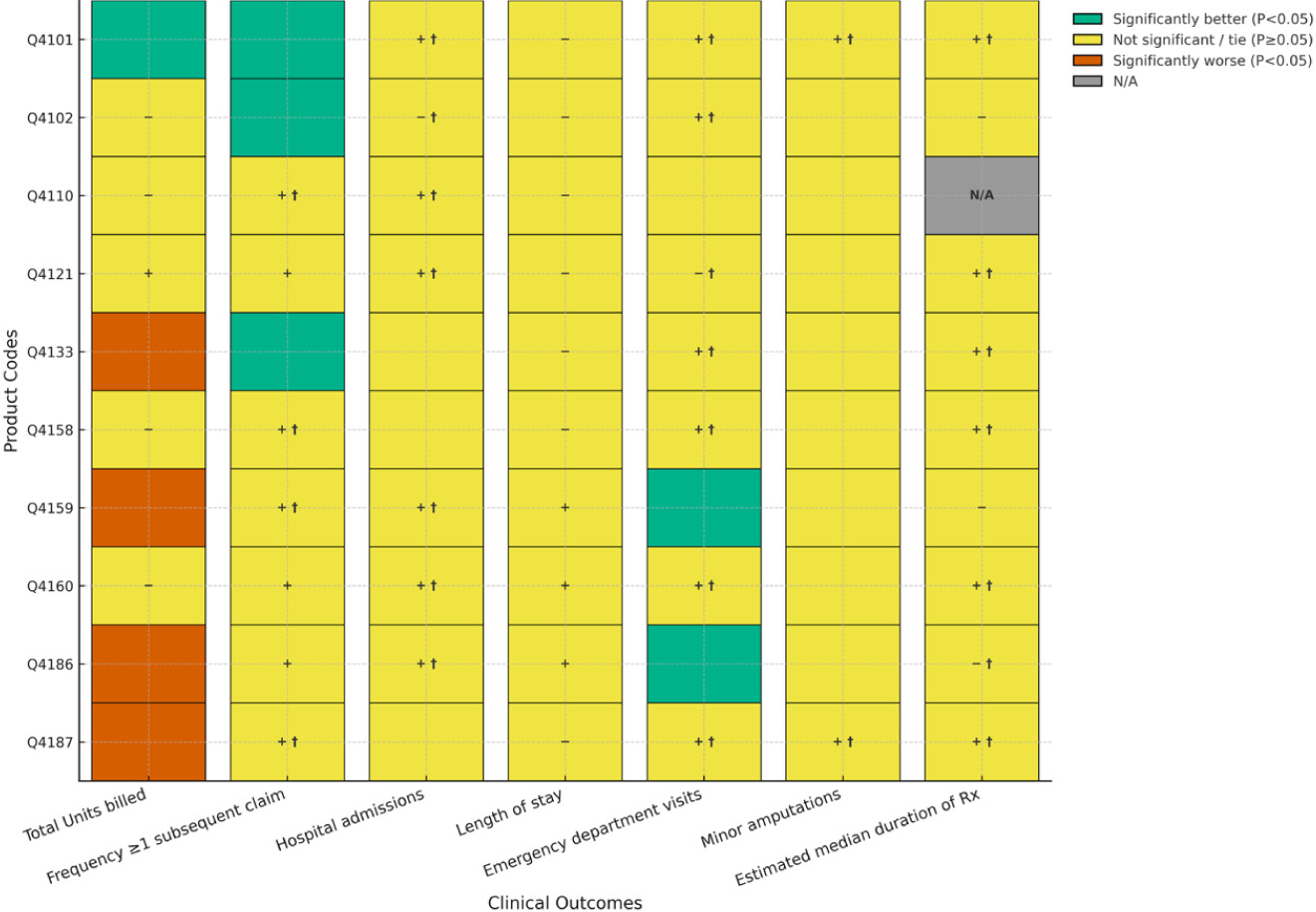
FIGURE 4 Comparisons between Cygnus Dual and matched covered products used for VLUs.
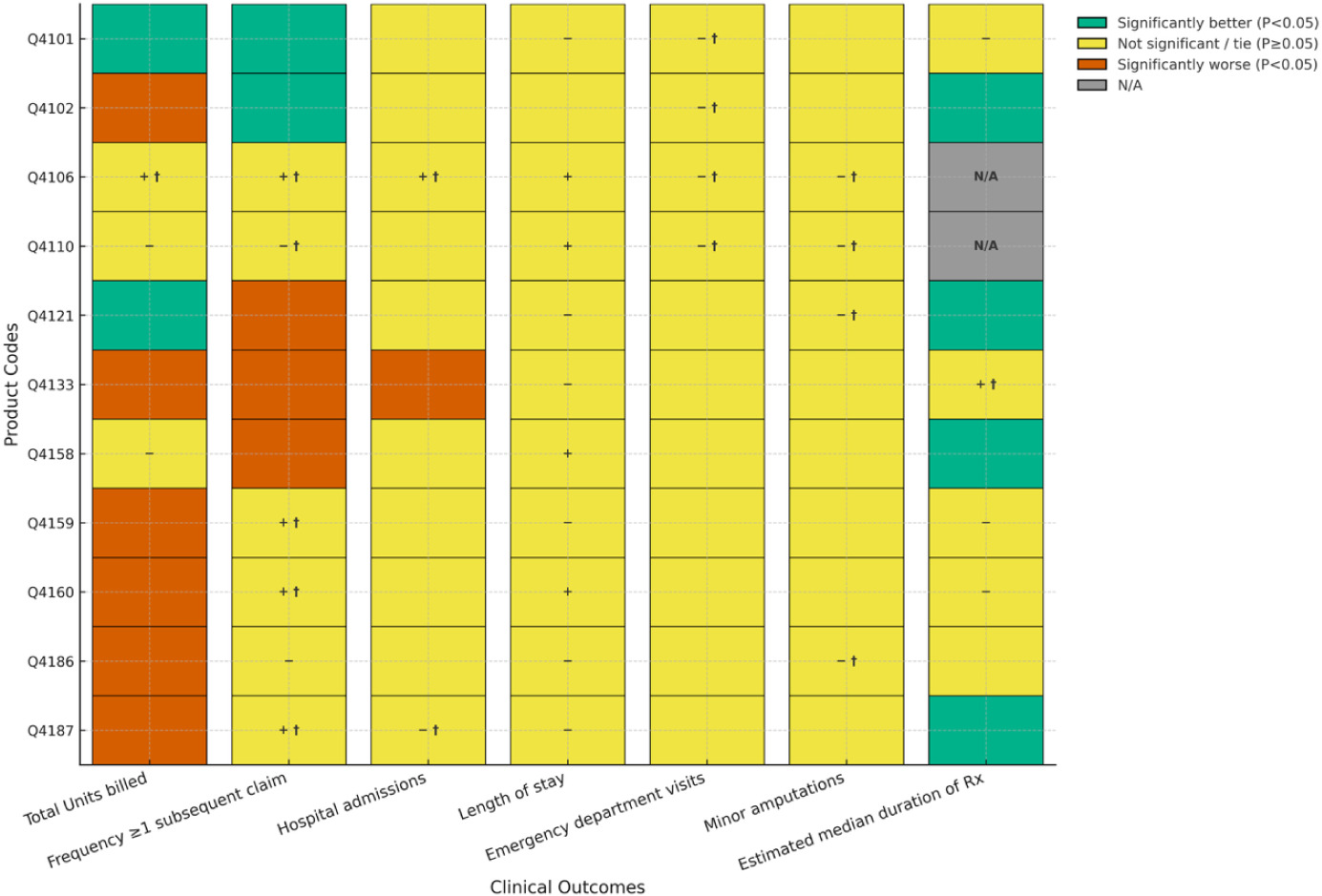
Figure 2 illustrates comparisons between Cygnus Dual and matched covered products used for DFUs. Treatment of DFUs with Cygnus Dual resulted in 6 of 11 (55%) product comparisons of average number of units billed significantly favoring the Cygnus Dual and 4 of 11 (36%) significantly favoring the comparator. The remaining product non-significant comparison did not achieve non-inferiority. For frequency of patients with ≥ 1 subsequent claim with a lower number of units billed, five comparisons (45%) showed statistical superiority for Cygnus Dual, and one comparison (9%) reached significance for one of the covered products. Overall, five comparisons were not significantly different, with all achieving non-inferiority. For hospitalizations, length of stay, and emergency department visits, only one comparison achieved statistical significance in favor of a covered product for emergency department visits with the remaining comparisons all being non-significant on this outcome. Comparisons between products for hospitalizations showed that two achieved noninferiority (with 9 ties). One comparison achieved noninferiority for length of stay. For minor amputations, two products (18%) were statistically superior to Cygnus Dual while comparisons on this outcome for the remaining 9 products did not achieve statistical significance with all being noninferior. For median duration of treatment, Cygnus Dual was statistically superior to 80% (8 of 10) of comparators with only one covered product (10%) showing statistical superiority and one product (10%) achieving non-inferiority.
TABLE 2 Comparison of clinical outcomes for DFU and VLU between Cygnus products and any covered product
|
|
DFU: Diabetic foot ulcer, VLU: Venous leg ulcer
Figure 3 illustrates comparisons between Cygnus Matrix and matched covered products used for VLUs. With respect to the treatment of chronic VLUs, Cygnus Matrix had significantly fewer units billed than one (10%) covered product and significantly more units billed than four (40%) covered products. The five remaining product comparisons did not achieve statistical significance with none achieving non-inferiority on this outcome. For frequency of patients with ≥ 1 subsequent claim with a lower number of units billed, three (30%) product comparisons statistically favored treatment with Cygnus Matrix with seven (70%) comparisons showing no statistically significant differences; four of these comparisons achieved non-inferiority. No covered product was significantly better than Cygnus Matrix on this outcome. For hospitalizations, length of stay, and emergency department visits, two (20%) comparisons resulted in significantly lower average number of emergency department visits for treatment with Cygnus Matrix. All other comparisons failed to reach statistical significance with most hospital and emergency department visits achieving non-inferiority between products. There were no statistically significant differences between Cygnus Matrix treatment and any covered product with respect to minor amputations, with two (20%) comparisons achieving non-inferiority. Similarly, there were no significant differences between products for median duration of therapy with 7 of 9 (78%) comparisons achieving noninferiority.
Figure 4 illustrates comparisons between Cygnus Dual and matched covered products used for VLUs. Treatment of VLUs with Cygnus Dual was associated with significantly fewer units billed than two of 11 (18%) covered products and a significantly higher number of units billed than six (55%) products. One of three nonsignificant comparisons achieved non-inferiority. For frequency of patients with ≥ 1 subsequent claim with a lower number of units billed, two (18%) comparisons statistically favored Cygnus Dual and three (27%) favored a covered product. Five of the remaining 6 comparisons achieved non-inferiority. All but one comparison, for hospital admissions statistically favoring a covered product, failed to achieve statistical significance for hospitalizations, length of stay, and emergency department visits. Non-inferiority was achieved for some nonsignificant comparisons for hospital admissions and emergency department visits (with many ties) but not length of stay. There were no statistically significant differences between Cygnus Dual treatment and any covered product with respect to minor amputations, with four of 11 (36%) comparisons achieving non-inferiority. Finally, Cygnus Dual was shown to be statistically superior to four of 9 (44%) covered products for median duration of therapy, with no comparator showing statistical superiority. One of five non-significant comparisons of this outcome achieved non-inferiority.
Table 2 provides comparisons on all clinical outcomes between both Cygnus products and all covered products combined for DFUs and VLUs. Over all 28 comparisons, six (21%) showed statistical superiority in favor of Cygnus, with 21 (75%) comparisons being non-significant and only one (4%) comparison favoring a covered product. Six non-significant differences between products achieved non-inferiority.
Discussion
Amnion-based skin substitutes, such as membranes from the human placenta, act as allogeneic biological scaffolds to facilitate wound healing. Amnion, being an ‘immune-privileged’ barrier, possesses anti-inflammatory, anti-scarring, and anti-microbial properties that promote regeneration without scarring.14 Due to the burgeoning number of amnion-and non-amnion-based skin substitute products being used for wound care, CMS has sought to establish consistency for the evaluation of new products by addressing evidence gaps that contribute to our overall understanding of the role of these products for wound care.
The current study used the Medicare administrative database to identify claim-related outcomes for products covered under the future effective LCDs compared to two Cygnus skin substitute products using direct 1-to-1 matching on important background variables. This methodology produced similar cohorts of patients to support valid comparisons between products on clinical outcomes of interest.
For both Cygnus products combined, the number of units billed to CMS was similar or significantly lower than comparative, covered products demonstrating fewer multi-claim trajectories. For example, Cygnus Matrix was associated with a similar (i.e., not statistically significantly different) or statistically superior reduction in the number of units billed, on average, compared to all 11 of the matched comparators for DFUs. Results were similar for Cygnus Dual where seven of 11 (64%) comparisons showed similarity or superiority in favor of the Cygnus product.
As an additional proxy for wound healing in DFUs, we compared the frequency of patients between groups who show ≥ 1 subsequent claim for the index procedure which included a lower number of units than billed on the index claim. For both Cygnus products combined, there was only one instance out of 22 possible comparisons, where a covered product showed statistical superiority on this outcome. In contrast, a Cygnus product showed statistical superiority in 9 comparisons.
The findings of fewer claims and lower per-claim unit intensity associated with the Cygnus products for DFUs translated to a generally lower median duration of therapy, with patients treated with Cygnus Dual, for example, showing a statistically significant reduction in therapy duration compared to 8 covered products.
Results for VLUs were similar. For both Cygnus products combined, 18 of 21 comparisons demonstrated statistical superiority or a non-significant difference in the frequency of patients who show ≥ 1 subsequent claim for the index procedure which included a lower number of units than billed on the index claim, a surrogate for progression in wound healing.
In many instances, non-significant differences between products achieved non-inferiority meaning that the Cygnus product was not worse than the covered comparator treatment by more than a pre-defined amount, known as the non-inferiority margin. Finally, inspection of Table 6 provides a visual display of the overall study findings when all covered products where combined providing robust sample sizes (e.g., >600 DFU, = 425 VLU) particularly for comparisons with Cygnus Dual. In this analysis, 27 of 28 (96%) comparisons across all clinical outcomes were similar or statistically superior in favor of a Cygnus product.
An extensive body of evidence exists throughout the literature to support the safety and effectiveness of placental-derived biomaterials in wound healing.14 The results of the current study, taken in toto, confirm similar or superior performance by the Cygnus products in comparison to an array of skin substitute products on the LCD covered lists with respect to claims-based clinical outcomes that reflect RWE regarding the management of chronic DFUs and VLUs. Such real-world performance supports their consideration in Medicare coverage decisions as clinically effective and economically responsible options for chronic wound management
Limitations
The primary limitations of using an RWE administrative database to assess comparative product effectiveness involves missing or incomplete data, coding inaccuracies, inconsistent data entry, and procedural changes over time. Most importantly, the data are primarily collected for billing and management, not for measuring clinical outcomes. We included several proxy measurements of wound healing, although the precision of this method is unknown.
Conclusion
This study aggregated claims-based clinical outcomes from the 100% Medicare database, providing a large body of RWE showing similar or superior performance by the Cygnus products in comparison to an array of skin substitute products on the LCD covered lists for treating DFUs and VLUs. Although this comparison applies a surrogate approach to critical metrics of healing such as wound size, depth, and closure, these restrictions are shared by all products on the covered list. Taken together, these findings provide robust real-world support for inclusion of Cygnus Matrix and Cygnus Dual in Medicare coverage determinations alongside currently listed products.