Introduction
Diabetic foot ulcers (DFUs) are a common and serious complication of diabetes, affecting up to one in four people with the disease during their lifetime. They are associated with prolonged morbidity, high rates of infection and amputation, and substantial health care costs. Standard of care (SOC) typically includes debridement, infection control, pressure offloading, and regular wound dressings, but many DFUs fail to heal under these measures alone. Cellular and/or tissue-based products (CAMPs) have emerged as adjunctive therapies designed to promote healing by providing structural scaffolds, growth factors, and regenerative cellular components. Although widely adopted in clinical practice, CAMPs are costly, and their coverage has become a focal point of payer policy.
In May 2023, all seven Medicare Administrative Contractors (MACs) jointly released proposed Local Coverage Determinations (LCDs)1 that substantially limited coverage for CAMPs used in the treatment of DFUs. Although issued under separate MAC jurisdictions, the LCDs were co-ordinated in scope, design, and evidentiary foundation. Each relied on a harmonized application of the Grading of Recommendations Assessment, Development and Evaluation (GRADE)2 framework to assess the strength of clinical evidence. After reviewing public comments, the MACs finalized the LCDs on January 4, 2024,3-8 establishing a unified national policy that restricted reimbursement to 17 specific CAMPs products based on 35 cited randomized controlled trials (RCTs).
While the LCDs provided a structured appraisal of evidence strength, the GRADE framework does not quantify effect sizes or account for statistical heterogeneity across trials. In particular, it leaves unanswered questions about the magnitude and consistency of CAMPs’ clinical benefit. The primary endpoint across all LCD-cited studies was complete wound closure, a clinically meaningful outcome that reflects full re-epithelialization, reduced infection risk, and lower likelihood of amputation. The LCDs synthesized this evidence using the GRADE framework to evaluate risk of bias, consistency, precision, and overall strength of recommendation. Yet differences in trial design, SOC definitions, and product characteristics complicate direct comparisons and reduce interpretability.
A rigorous quantitative synthesis is therefore needed to complement the GRADE assessments. Conventional fixed-effects or frequentist meta-analyses are ill-suited to this evidence base given its heterogeneity and missing comparators. By contrast, a Bayesian hierarchical beta-binomial model allows for partial pooling across studies, accommodates overdispersion in healing outcomes, and can generate probabilistic predictions for trials lacking SOC arms.9 This approach to the meta-analysis of binary outcomes was discussed at length by Zhang,10 and performed inter alia by Spiegelhalter.11 This approach provides both product-specific and pooled effect estimates while directly quantifying uncertainty.
In this study, we conducted a Bayesian meta-analysis of the 35 RCTs cited in the 2024 Medicare LCDs. Our objective was not to re-evaluate the full CAMPs literature, but to quantify the evidence already deemed sufficient for policymaking. By doing so, we provide a transparent statistical interpretation of the LCDs and establish benchmarks for clinicians, sponsors and payers in evaluating CAMPs-based therapies.
Methods
Study identification
We identified studies based on the list of RCTs (depicted in Figure 1) published by all seven MACs. These LCDs listed 35 RCTs supporting coverage for 17 specific CAMPs products. Each LCD referenced the same harmonized set of studies, derived from a co-ordinated GRADE assessment process.
No additional database search (e.g., MEDLINE, Embase) was performed beyond this pre-specified source. This decision reflects our objective: to formally quantify the evidence base used by the MACs in determining coverage. To ensure accuracy and completeness, we extracted publication identifiers (PMIDs, DOIs), journal names, study arms, and outcome data directly from the LCDs and cross-referenced them with the original publications accessed via PubMed and publisher websites.
This focused search strategy supports the analytic goal of replicating the evidentiary foundation already judged sufficient for Medicare coverage, rather than re-assessing the broader body of CAMPs literature. By restricting inclusion to LCD-cited trials, we avoided selection bias and maintained alignment with the policy-relevant evidence base.
Classification of trials, outcomes, and definitions
As shown in Figure 2, trials were classified based on design characteristics reported in the LCD source document, original trial publications, and supplementary materials when available. Most studies were two-arm RCTs comparing CAMPs to SOC over a 12-week follow-up. The sample also included single-arm CAMPs studies, three-arm trials, and trials with longer durations (e.g., 16 or 20 weeks).
The primary outcome across all studies was complete wound closure, typically defined as 100% re-epithelialization with no drainage at the index wound site. While wound assessment protocols varied, the healing definition was standardized and consistently extracted from the LCDs. When multiple endpoints or time points were reported, we selected the final follow-up window listed in the LCDs.
FIGURE 1 List of 35 RCTs supporting coverage for 17 specific CAMPs products published by all seven MACs.
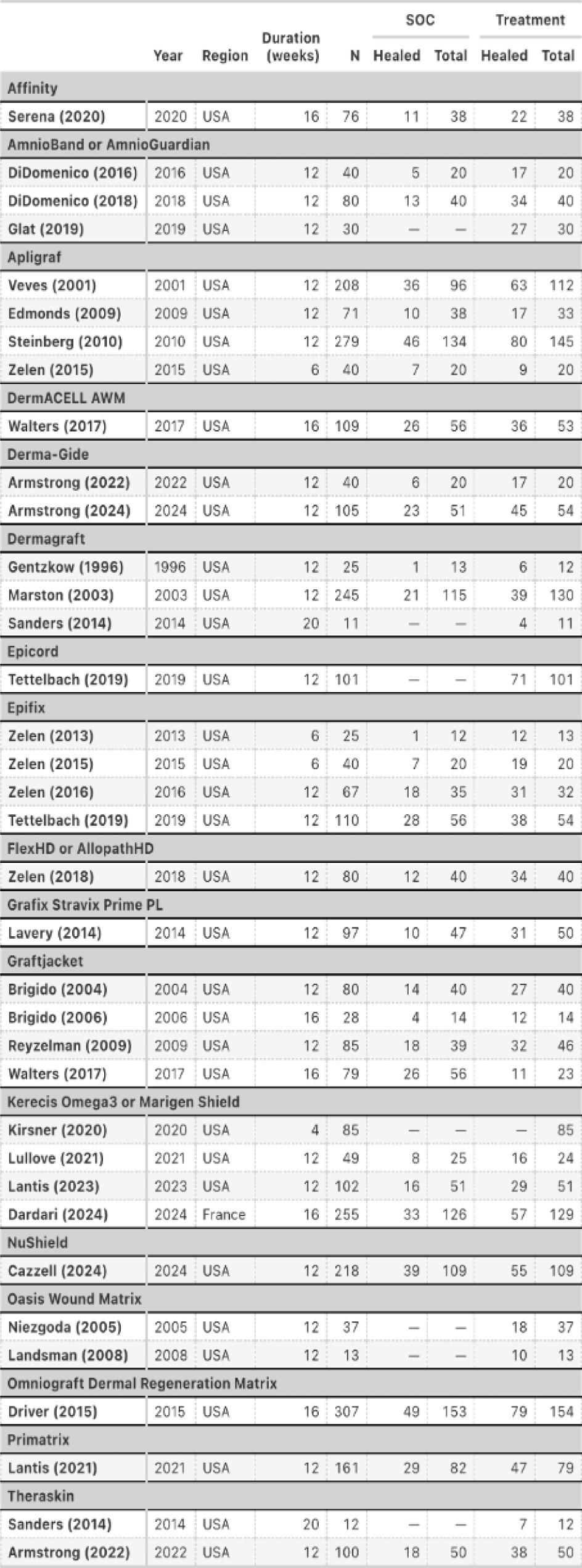
Each study arm was classified by treatment type (CAMPs or SOC) and by product. Outcomes were recorded as the number of healed wounds out of the number treated. This enabled arm-level modeling and partial pooling across studies while accounting for variability in product, comparator, and trial design.
FIGURE 2 Trials were classified based on design characteristics reported in the LCD source document, original trial publications, and supplementary materials when available.
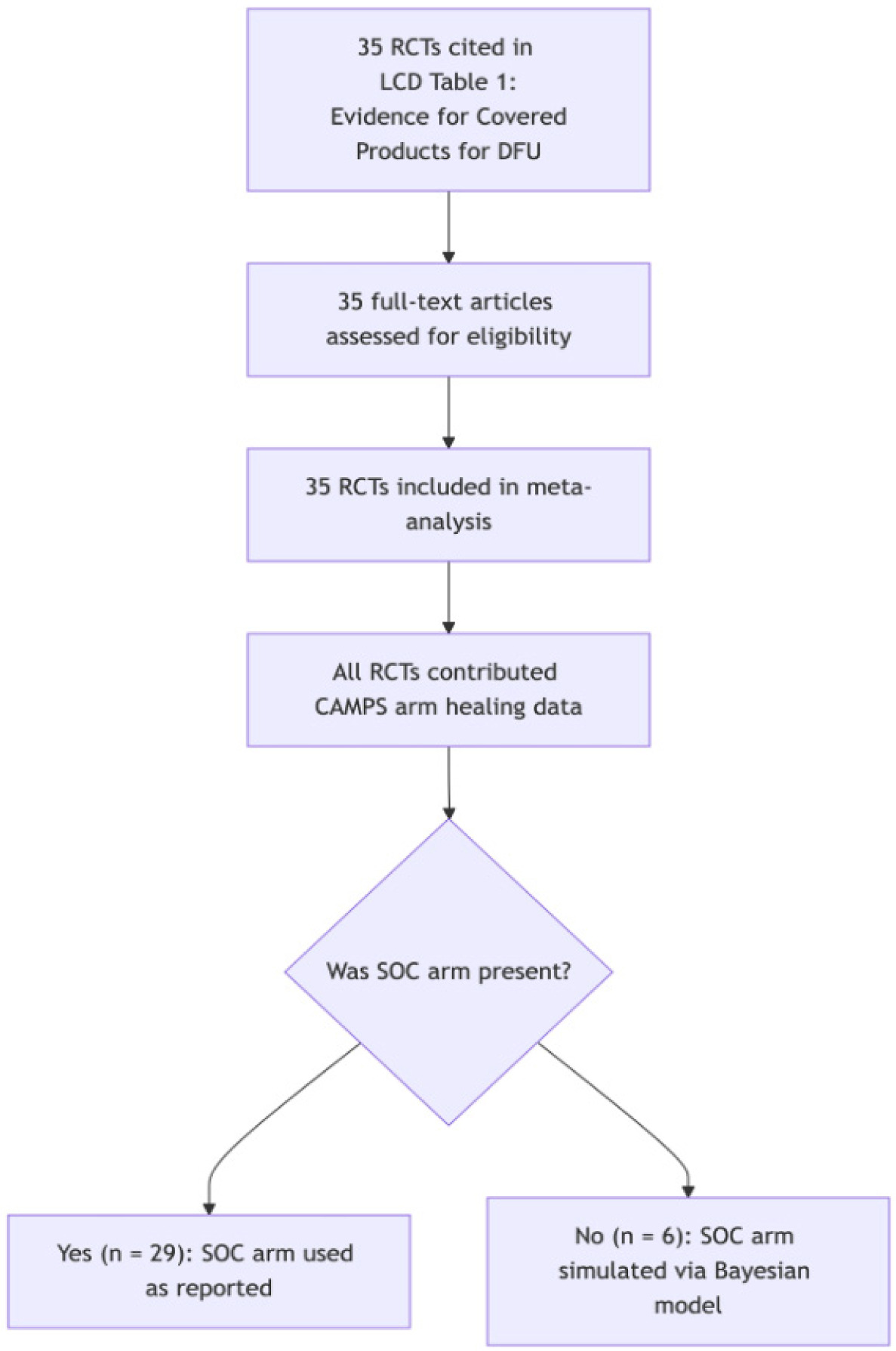
This classification ensured fidelity to the policy-relevant evidence base and supported hierarchical modeling of binomial outcomes across a diverse set of trial designs.
Statistical methods
Model structure
The authors modeled wound healing outcomes using a Bayesian hierarchical beta-binomial regression. The unit of analysis was the study arm, with each arm contributing the number of wounds healed out of the total treated. To account for greater-than-binomial variation (i.e., heterogeneity across patients, protocols, or measurements), a beta-binomial likelihood was used.
In this model, μ_i represents the average healing probability in arm (i), and κ_i captures how tightly individual outcomes cluster around the average. A higher κ implies more consistency; a lower κ allows for greater within-arm variation.
Healing probability was modeled on the logit scale. The model included an overall intercept (α) representing baseline healing across arms, a fixed effect of follow-up time in weeks (β∙weeki), and random effects to account for differences by treatment arm (CAMPs versus SOC), by product, and by product-treatment combinations.
This structure accounts for differences in product performance and allows these effects to vary between CAMPs and SOC arms. The use of random effects enables uncertainty in product-specific performance while leveraging shared information across studies (‘partial pooling’).
We also allowed variability (κi) within each arm to differ by product and treatment type, reflecting the fact that some studies reported more consistent healing outcomes than others. This ensures that results from arms with wide variability are appropriately down weighted in the final estimates.
By using a hierarchical model, we accommodate between-trial variation, estimate plausible outcomes for missing comparators, such as SOC arms in single-arm trials, and strengthen inference by borrowing information across related study conditions.
Estimand and effect measures
The estimand was defined in log-odds space and transformed to healing probability using the expit (inverse logit) function. Risk ratios were calculated by dividing the predicted probability of complete wound closure in the CAMPs arm by the predicted probability in the SOC arm.
Priors
In Bayesian analysis, priors reflect initial assumptions about model parameters.12 They help stabilize estimates when data are sparse or heterogenous. We used weakly informative priors to define plausible ranges for healing rates and between-study variation, without overpowering the observed data.
-
Broad priors were applied to the intercept (baseline healing rate) and time effect, allowing for wide variation in healing rates and modest increases or decreases over time.
-
The prior on the time effect was centered at zero to reflect uncertainty about whether longer follow-up improves outcomes.
-
Random effects were modeled with priors on both effect sizes and variances, permitting product-specific variation but limiting extreme inferences unless strongly supported by data.
-
Dispersion priors allowed some arms to show more consistent outcomes than others, an important feature when synthesizing studies with variable design quality and sample size.
To assess the robustness of the treatment effect to prior assumptions, three Normal prior distributions were specified on the log scale for both baseline healing and treatment effects. First, a diffuse prior was used to represent minimal prior information. Second, a skeptical prior was parameterized to correspond to a mean 30% healing probability on the outcome scale for both the treatment and SOC arms. Third, an informative prior reflecting a mean 30% healing probability for SOC and mean 50% for the treatment arm was applied. These priors were selected to span a range of prior beliefs: ignorant, skeptical, and optimistic. Sensitivity analyses indicated that posterior estimates were minimally affected by prior specification, suggesting that the data and likelihood were sufficiently informative to dominate the prior distributions.
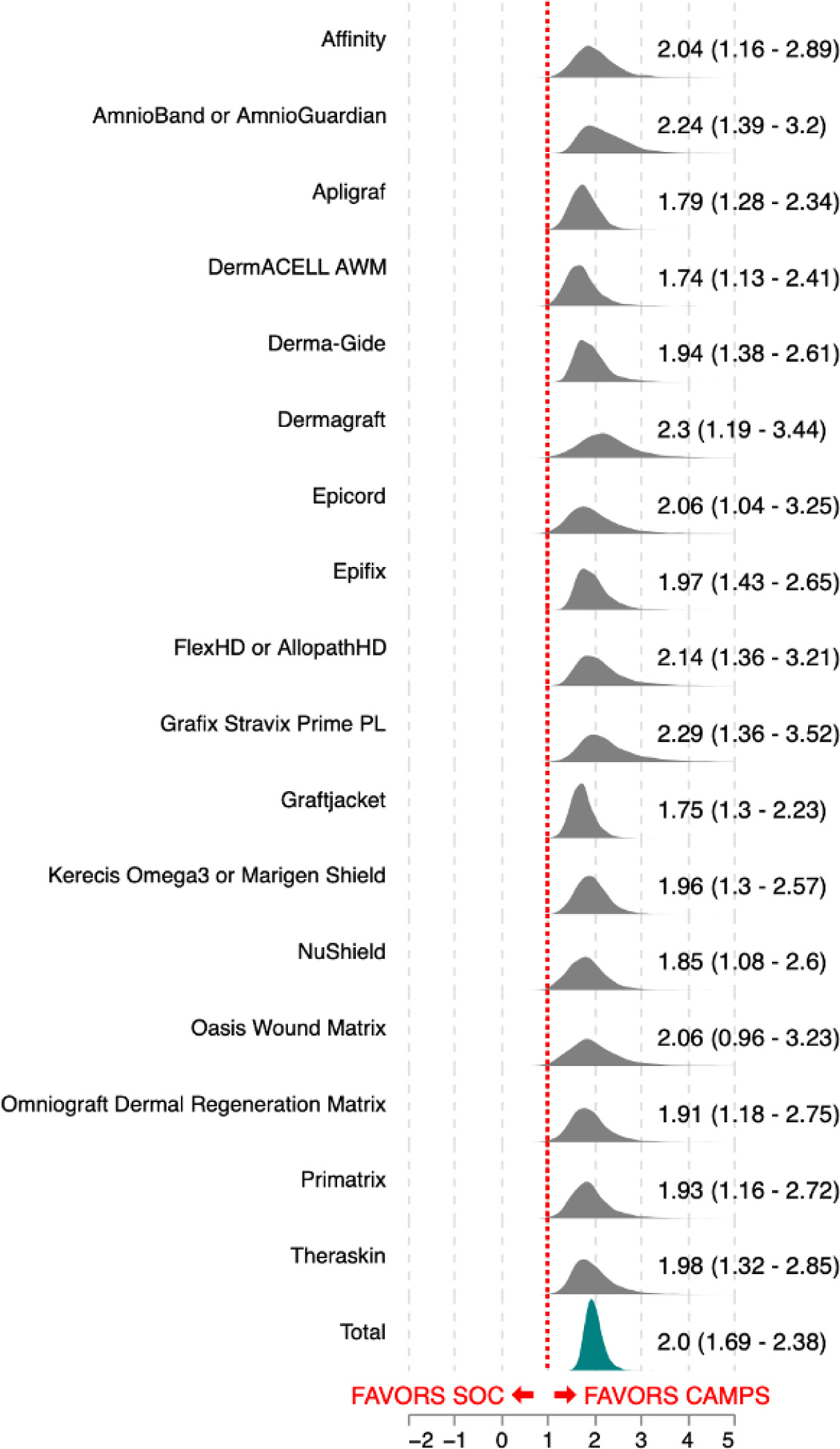
FIGURE 3 Product-level posterior distributions of risk ratios, depicted as shaded density curves. Wider intervals correspond to fewer contributing trial arms or greater variability across study results, whereas narrower intervals reflect more consistent performance across multiple trials.
Standardization and estimation of effects
Healing probabilities were estimated for each treatment arm of each product by sampling from the posterior predictive distribution of the hierarchical beta-binomial model. To facilitate cross-study comparison, we standardized all predictions to a 12-week follow-up and a hypothetical sample size of 100 patients (50 per arm). This standardization reflects the most common LCD trial duration and provides an interpretable benchmark scale for comparing healing rates across heterogeneous studies.
Product-specific risk ratios were then calculated as the ratio of healing probability in the CAMPs (treatment) arm to that in the SOC arm. These estimates reflect marginal effects derived from the posterior, incorporating both within- and between-study uncertainty. A pooled risk ratio summarizing the overall treatment effect was also calculated from the population-level posterior.
Posterior predictions were additionally used to simulate outcomes for trials lacking SOC comparators (e.g., single-arm CAMPs studies), allowing inclusion of all LCD-cited trials without naïve imputation or exclusion.
Results
Across all 35 LCD-cited RCTs, the pooled posterior mean risk ratio for CAMPs versus SOC was 2.00 (95% CrI: 1.69–2.28). This indicates that, on average, wounds treated with a CAMPs product were approximately twice as likely to achieve complete closure as those treated with SOC alone.
All 17 products included in the analysis demonstrated improved healing rates relative to SOC. Product-specific posterior distributions showed minimal overlap with a risk ratio of 1.0, providing consistent evidence in favor of CAMPs treatment across all products evaluated.
At the individual product level, estimated posterior mean risk ratios ranged from 1.74 to 2.30. For every product, the posterior probability mass near the null effect (risk ratio = 1.0) was negligible, supporting a consistent direction of effect, though the magnitude varies modestly across products. While a few products showed small tails of their posterior distributions extending near 1.0, the probability of no effect was less than 3% in every case.
Figure 3 presents product-level posterior distributions of risk ratios, depicted as shaded density curves. Wider intervals correspond to fewer contributing trial arms or greater variability across study results, whereas narrower intervals reflect more consistent performance across multiple trials.
Taken together, these findings support a robust and reproducible treatment benefit for CAMPs across a diverse range of study designs, comparators, and patient populations. The standardized predictions (12-week follow-up, 100 patients per trial) facilitate direct comparisons across heterogeneous RCTs and reinforce the conclusion that CAMPs substantially improve healing relative to SOC.
Discussion
This Bayesian meta-analysis synthesizes wound healing outcomes from the 35 RCTs cited in the 2024 Medicare LCDs for CAMPs products. Using a hierarchical beta-binomial model, we estimated both product-specific and pooled treatment effects relative to SOC. Our findings indicate that CAMPs consistently improve wound closure rates when compared with SOC alone.
The pooled posterior mean risk ratio for CAMPs versus SOC was 2.00 (95% CrI: 1.69–2.38), indicating that wounds treated with CAMPs were approximately twice as likely to heal as those receiving SOC. Product-specific analyses supported this conclusion, with all 17 products demonstrating superior healing relative to SOC. Posterior probability mass near the null effect was negligible in every case, suggesting high certainty in the direction of benefit. These results were derived from standardized posterior predictions simulating a typical trial scenario: 12 weeks of follow-up and 100 randomized patients (50 per arm). Standardization allowed direct comparisons across heterogeneous trial designs and ensured fair interpretation of treatment effects. By modeling outcomes at the arm level, we incorporated both within- and between-study uncertainty, leveraged partial pooling across studies, and generated plausible SOC comparators for single-arm trials.
The hierarchical beta-binomial framework was particularly well-suited for this evidence base. It accommodates variability in baseline healing rates, adjusts for overdispersion, and shrinks extreme estimates toward the global mean while preserving product-specific effects. Importantly, Bayesian inference allowed us to present results as posterior probabilities rather than relying solely on confidence intervals, providing a more intuitive interpretation of uncertainty for clinicians and policymakers.
This analysis complements, rather than replaces, the GRADE assessments used in the LCD process. While GRADE provides a structured, qualitative appraisal of risk of bias and strength of evidence, our meta-analysis delivers a formal quantitative estimate of effect size and its uncertainty. Together, these approaches provide a transparent and policy-relevant interpretation of the evidence underpinning Medicare‘s 2024 coverage decisions.
Limitations
As with any meta-analysis, our conclusions are constrained by the quality of the studies included. Although the hierarchical model accounts for heterogeneity and missing comparators, residual confounding or publication bias—particularly given the prevalence of industry-sponsored CAMPS trials—may still influence results. Standardizing predictions to a 12-week follow-up improves comparability but does not capture longer-term trajectories or variability in real-world SOC practices. Furthermore, the model assumes exchangeability of trials once design and product factors are incorporated; unmeasured differences in patient populations or clinical practice may nonetheless affect healing outcomes.
Conclusion
For clinicians, this analysis provides robust product-specific estimates of expected healing outcomes under typical trial conditions. For sponsors, the results clarify the treatment effects associated with currently covered CAMPs products and offer practical benchmarks for trial design and positioning. For regulators and payers, the findings present a reproducible and transparent quantification of treatment benefit, derived from the same evidence base that informed Medicare’s 2024 LCDs. By anchoring interpretation to the LCD-cited RCTs, this analysis strengthens confidence in the policy foundation and provides a framework for evaluating future CAMPs interventions.