Introduction
Type 2 diabetes mellitus (T2DM) represents the predominant form of diabetes, comprising the majority of the over 500 million cases globally.1 Its growing prevalence is largely driven by modifiable risk factors such as obesity, poor metabolic health, sedentary behavior, tobacco use, and alcohol consumption.2 One of its most debilitating complications is the development of diabetic foot ulcers (DFUs), which result from factors such as excessive plantar pressure, ill-fitting footwear, abnormal gait, and repetitive trauma.3 Evaluation and classification of DFUs typically take into account ulcer size and depth, severity of infection, presence of peripheral neuropathy or peripheral arterial disease, and anatomical location.4
DFUs occur in an estimated 19–34% of individuals with diabetes and are linked to a five-year mortality rate ranging from 50% to 70%.3 Approximately 20% of moderate to severe DFUs ultimately progress to foot amputation.5 Over the past decade, the annual rate of DFU-related amputations has risen from 1.5 to 3.5 cases per 1,000 patients with diabetes.6-9 The standard of care (SOC) for DFUs consists of sharp debridement, offloading, controlling bacterial burden, and maintaining moisture balance. Despite these measures, SOC achieves closure in fewer than 50% of DFUs within 12 weeks.10 Routine screening, typically involving the combination of primary and specialty care providers, serves as a primary preventive approach, though it incurs considerable healthcare costs.11 In the United States, diabetic foot disease was estimated to impose an economic burden of approximately $80 billion in 2017.9 Patients with lower socioeconomic status, especially those in minority populations, experience markedly worse outcomes, largely due to limited access to advanced wound care services.12 Although chronic wounds pose a major clinical and financial burden, research in this area receives minimal funding—only 0.17% of the $7 billion distributed by the U.S. National Institutes of Health between 2002 and 2011 was directed toward DFU studies.13
Advanced wound care includes the use of cellular, acellular, and matrix-like products (CAMPs), which encompass a wide range of biomaterials, synthetic substances, and biosynthetic matrices designed to aid tissue repair and regeneration through multiple mechanisms of action.14 CAMPs provide several therapeutic advantages, such as shielding the wound environment, covering exposed underlying tissues, supporting surgical closure, and enhancing both functional recovery and aesthetic outcomes.14 Within the cellular category, allografts, human donor tissues used for transplantation, have shown strong potential as biologically active materials that promote DFU healing by providing structural support and exhibiting anti-inflammatory and regenerative effects.15
The CAMPAIGN clinical trial employed a modified master trial design, platform, to evaluate multiple lyophilized human amnion/chorion membrane (LHACM) products within a single overarching protocol. The full study protocol for this platform design was published in 2025.16 The initial phase will include two HACMs, one of which is included in this interim analysis. The platform design provides flexibility for addition or removal of products based on analysis of data.
Materials and methods
CAMPAIGN is a multicenter, prospective, randomized controlled modified platform trial designed to determine the difference in achieving complete closure of nonhealing diabetic foot ulcers (DFUs) with multiple LHACMs plus SOC versus SOC alone over 12 weeks using a modified platform trial design (clinicaltrials.gov #NCT06600724). The protocol initially specifies two LHACMs for evaluation; however, the adaptive nature of the platform design permits the addition of new investigational products contingent upon data analysis and protocol amendments. The interim analysis evaluated one of the two planned test products. This study was conducted at 12 SerenaGroup®, Inc. or affiliated centers throughout the United States with 71 patients with nonhealing DFUs. Enrollment for this study began October 2024 and interim analysis was conducted September 2025. The study population was drawn from patients suffering from DFUs who were attending wound clinics.
Objectives and endpoints
The primary objective for the CAMPAIGN clinical trial was to determine the difference in achieving complete closure of nonhealing DFUs with multiple LHACMs plus SOC versus SOC alone over 12 weeks using a modified platform trial design. The primary endpoint was the percentage of target ulcers achieving complete wound closure in 12 weeks. An additional important endpoint evaluated was percentage wound area reduction (PAR) from TV-1 to TV-13 measured weekly with digital photographic planimetry, using a digital imaging device, and physical examination.
Diagnosis
Diagnosis of DFUs is primarily clinical, based on a detailed medical history, comprehensive physical examination, and selective use of diagnostic tests. These ulcers most often occur on weight-bearing regions of the foot and are commonly associated with peripheral neuropathy and peripheral arterial disease (PAD). Neuropathic ulcers typically feature a callused border, a well-defined appearance, and variable granulation tissue, while ischemic or neuroischemic ulcers tend to exhibit irregular edges, pale or necrotic bases, and limited exudate. Sensory neuropathy often diminishes or eliminates pain sensation, although discomfort may increase in the presence of ischemia or infection.
A comprehensive clinical history is crucial for distinguishing DFUs from other types of chronic wounds. Important aspects include the duration of diabetes and level of glycemic control, history of previous ulcers or amputations, presence of peripheral vascular disease, neuropathic symptoms, mechanical or traumatic injury mechanisms, prior wound care interventions, and footwear practices. Differential diagnoses to rule out include venous leg ulcers, non-diabetic arterial ulcers, pressure injuries, vasculitic lesions, and malignant ulcers.
A bedside neurological assessment was conducted to determine the presence or absence of protective sensation. All prospective participants also underwent vascular evaluation, with the ankle-brachial index (ABI) serving as the primary screening tool. Individuals with an ABI greater than 0.7 were considered eligible, while those with values above 1.3 required further assessment. For patients with noncompressible or calcified arteries, alternative tests such as the toe-brachial index (TBI) were used, where values of 0.6 or higher indicated sufficient perfusion. Additionally, a transcutaneous oxygen measurement (TCOM) of 40 mmHg or above was accepted as evidence of adequate blood flow for inclusion.
Vulnerable populations
Although vulnerable subjects were not specifically recruited for this study, vulnerable subjects were present in the potential subject pool. According to the U.S. Food and Drug Administration (FDA), vulnerable subjects are individuals who are at an increased risk,either physically, psychologically, socially, or economically,of being coerced or unduly influenced into participating in clinical research
Product description
This study evaluated two products: lyophilized human amnion/chorion membrane (LHACM; EPIEFFECT®, MIMEDX Group, Inc., Marietta, GA, USA) and another amniotic product to be later determined. In keeping with the platform design, additional products may be added at a later date. Only one LHACM was evaluated in the interim analysis. LHACM is a tri-layer allograft composed of the placental amnion, intermediate, and chorion layers, an extracellular matrix (ECM) forming a protective barrier that supports natural healing. The allograft thickness simplifies application and repositioning after hydration.17 It has been used in combination with various wound management treatments, including compression therapy, negative pressure wound therapy (NPWT), and hyperbaric oxygen therapy. Its five-year shelf life and terminal sterilization process enhance safety and convenience for healthcare professionals.17
Subject characteristics
Patients who suffer from nonhealing diabetic foot ulcers were recruited for this study from participating wound clinics. Once patients agreed to adhere to the study schedule, and read and signed the IRB approved Informed Consent Form, screening was conducted to determine whether subjects were eligible based on inclusion and exclusion criteria, listed in Table 1.
TABLE 1 Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Must be at least 18 years of age or older. • Must have diagnosis of type 1 or 2 Diabetes mellitus. • At randomization, must have a target ulcer with a minimum surface area of 0.7 cm2 and a maximum surface area of 20.0 cm2 measured post debridement. • Must have been present for a minimum of 4 weeks with standard of care, prior to the initial screening visit. • Must be located on the foot with at least 50% of the ulcer below the malleolus. • Must be Wagner 1 or 2 grade, extending at least through the dermis or subcutaneous tissue and may involve the muscle provided it is below the medial aspect of the malleolus. May include exposed muscle, tendon, or bone. • Must have adequate perfusion confirmed by vascular assessment. Any of the following methods performed within 3 months of the first screening visit are acceptable: o ABI between 0.7 and ≤ 1.3; o TBI ≥ 0.6; o TCOM ≥ 40 mmHg; o PVR: biphasic. • If two or more ulcers, must be separated by at least 2 cm. The largest ulcer satisfying the inclusion and exclusion criteria will be designated as the target ulcer. • Target ulcers located on plantar aspect of foot must be offloaded for at least 14 days prior to enrollment. • Must consent to using the prescribed offloading method for the duration of the study. • Must agree to attend the weekly study visits required by the protocol. • Must be willing and able to participate in the informed consent process. |
• Known to have a life expectancy of < 6 months. • Target ulcer is not secondary to diabetes. • Target ulcer is infected or there is cellulitis in the surrounding skin. • Target ulcer exposes tendon or bone. • Evidence of osteomyelitis complicating the target ulcer. • Infection in the target ulcer or in a remote location that requires systemic antibiotic therapy. • Receiving immunosuppressants (including systemic corticosteroids at doses greater than 10 mg of prednisone per day or equivalent) or cytotoxic chemotherapy or is taking medications that the PI believes will interfere with wound healing (e.g., biologics). • Taking hydroxyurea. • Has applied topical steroids to the ulcer surface within one month of initial screening. • Has a previous partial amputation on the affected foot that results in a deformity that impedes proper offloading of the target ulcer. • Has glycated hemoglobin (HbA1c) greater than or equal to 12% within 3 months of the initial screening visit. • Has reduced in size by more than 20% in the 2 weeks prior to the initial screening visit ('"historical'" run-in period). • Surface area measurement of the target ulcer decreases by 20% or more during the 2-week screening phase: the 2 weeks from the initial screening visit (S1) to the TV-1 visit during which time the potential subject received SOC. • Has an acute Charcot foot, or an inactive Charcot foot, which impedes proper offloading of the target ulcer. • Pregnant or considering becoming pregnant within the next 6 months are excluded. • Has end stage renal disease requiring dialysis. • Participation in a clinical trial involving treatment with an investigational product within the previous 30 days. • Has a medical or psychological condition that may interfere with study assessments. • Was treated with hyperbaric oxygen therapy (HBOT) or a Cellular, Acellular, Matrix-like Product (CAMP) in the 30 days prior to the initial screening visit. • Has a malnutrition indicator score <17 as measured on the Mini Nutritional Assessment. • Has a wound with active or latent infection is excluded. • Has a disorder that would create unacceptable risk of post-operative complications. • Has a known sensitivity to aminoglycoside antibiotics. |
Study procedures
Participants underwent a structured sequence of clinical visits including screening, treatment, healing confirmation, and follow-up phases to ensure accurate eligibility assessment, standardized wound care, consistent intervention delivery, and reliable endpoint determination. Subjects were evaluated weekly (± 3 days) over a 12-week treatment period, with any additional dressing changes recorded as unscheduled visits and abbreviated assessments performed when needed.
Participants who did not meet eligibility criteria at initial screening but were subsequently determined eligible were re-consented and assigned a new screening number. Up to three screening attempts were allowed, and those who subsequently met all inclusion and no exclusion criteria were enrolled. At the screening visit, conducted approximately 14 days prior to enrollment, informed consent was obtained, followed by a review of medical history to assess eligibility based on inclusion and exclusion criteria. Demographic data (including height, weight, BMI, gender, and ethnicity), medical and medication histories, and current use of non-steroidal anti-inflammatory drugs (NSAIDs) and opioids were recorded.
A vascular screening test was performed unless recent results (≤3 months) were available. Vital signs were measured, and a general physical examination was conducted.
Additional assessments included the Mini Nutritional Assessment (MNA), HbA1c testing (unless available within 3 months), and condition-specific evaluations: Wagner grade, Fitzpatrick skin type, pain intensity via Pain, Enjoyment of Life and General Activity Scale (PEG), and detailed wound characterization (granulation tissue, nonviable tissue, depth, exudate, and periwound skin). Historical wound measurements from two weeks prior to screening were collected; a reduction in wound size of >20% during this historical run-in period resulted in screen failure.
During the two-week active screening phase, standard-of-care (SOC) wound management was provided, consisting of cleansing with normal sterile saline (NSS), sharp debridement, post-debridement ulcer photography and measurement using a digital imaging device, and application of calcium alginate or foam dressing. Patients also initiated trial-specific offloading with a total contact cast (TCC), with four weeks of documented offloading required before enrollment.
At the enrollment/randomization visit (Treatment Visit (TV) 1; Day 0), eligibility was reconfirmed, adverse events and medication changes were reviewed, and a symptom-directed physical examination was performed. The percentage area reduction (PAR) from the screening period was assessed to confirm it remained <20%. Vital signs, wound characteristics, PEG pain score, Forgotten Wound Score (FWS), and Wound Quality of Life (wQOL) questionnaires were completed. Eligible participants were randomized to receive either LHACM with SOC or SOC alone. Wounds were cleansed with NSS, debrided, photographed, and measured using a digital imaging device before treatment. Dressings were applied as per protocol, with optional absorptive dressings for highly exudative wounds upon medical monitor approval. Patients not using TCC were assessed for adherence to offloading.
Participants returned weekly (TV-2 to TV-12) for safety and efficacy monitoring, including review of adverse events, medication changes, vital signs, wound assessment, pain scoring, and questionnaire administration (FWS and wQOL). Wound cleansing, debridement, measurement, and treatment per randomization arm were repeated at each visit. At the final treatment visit (TV-13) or earlier if wound closure occurred, adverse events, medication changes, pain, and quality-of-life measures (FWS, wQOL) were recorded. For unhealed ulcers, wound characteristics were documented, and follow-up care was arranged.
Healing was confirmed at a dedicated visit 14 ± 3 days after the first observation of complete re-epithelialization without drainage. This visit included adverse event review, medication update, pain assessment, investigator confirmation of closure, ulcer photography and measurement, and independent blinded verification. Early withdrawals underwent procedures equivalent to the final visit when possible. Unscheduled visits were conducted as needed for adverse event evaluation, medication review, and dressing changes.
At study exit, participants with unhealed wounds were transitioned back to physician-directed SOC. Independent healing confirmation was performed by two blinded wound care specialists reviewing de-identified digital images from closure and confirmation visits. Disagreements between wound reviewers were resolved in favor of the assessment aligning with the principal investigator's determination. Table 2 details the schedule of events for the study.
TABLE 2 Study schedule
| SV | TV-1 | TV-2, TV-3 | TV-4, TV-5 | TV-6, TV-7 | TV-8, TV-9 | TV-10, TV-11 | TV-12 | TV-13 | CCV | |
|---|---|---|---|---|---|---|---|---|---|---|
| Window Period | -14 | Day 0 | Week 1, Week 2 | Week 3, Week 4, | Week 5, Week 6 | Week 7, Week 8, | Week 9, Week 10 | Week 11 | Week 12 | +14 |
| Record medical history and demographic information | X | |||||||||
| Assessment of eligibility | X | X | ||||||||
| Sign informed consent form | X | |||||||||
| Vascular screening test | X | |||||||||
| Physical exam | X | X | ||||||||
| Mini Nutrition Assessment (MNA) | X | |||||||||
| HbA1c | X | |||||||||
| Wagner grade | X | |||||||||
| Fitzpatrick scale | X | |||||||||
| Historical measurement | X | |||||||||
| Randomization | X | |||||||||
| Assessment for AE and SAE | X | X | X | X | X | X | X | X | X | |
| Review medication for changes | X | X | X | X | X | X | X | X | X | |
| Vital signs | X | X | X | X | X | X | X | X | ||
| Wound assessment | X | X | X | X | X | X | X | X | X | X |
| Pain assessment (PEG) | X | X | X | X | X | X | X | X | X | X |
| wQOL | X | X | X | X | X | X | X | X | ||
| FWS | X | X | X | X | X | X | X | X | ||
| Study ulcer cleaning, debridement (if applicable) | X | X | X | X | X | X | X | X | X | |
| Study ulcer area with | ||||||||||
| digital images | X | X | X | X | X | X | X | X | X | X |
| Treatment based on | ||||||||||
| randomization | X | X | X | X | X | X | X | |||
| Apply dressing | X | X | X | X | X | X | X | X | X | |
| Offloading | X | X | X | X | X | X | X | X |
All participants had the right to withdraw from the study at any time during the treatment period without prejudice. The completion status of each participant's involvement in the clinical trial was documented. In the event that the study treatment or protocol-required observations were discontinued for any participant, the reason(s) for discontinuation were recorded. The investigator had the authority to withdraw a participant from the study at any time if deemed medically necessary. Whenever feasible, the reason for withdrawal or early termination was documented.
A participant was classified as lost to follow-up if they could not be reached after five telephone contact attempts and three written communications.
Subject compensation
Participants received a nominal compensation of USD 50 upon completion of each study visit. This compensation was intended to offset expenses associated with participation, including travel, parking, and the additional time required for study-specific procedures and data collection.
Statistical appendix
To meet contemporary Bayesian reporting and reproducibility standards, as outlined in the 'FDA 2010 Guidance for the Use of Bayesian Statistics in Medical Device Clinical Trials', a Statistical Appendix is provided.
Results
A total of 71 DFU patients from 12 sites were included in the interim analysis. 21 patients received standard of care alone and 50 patients received LHACM with SOC. Baseline characteristics are shown in Table 3. A Bayesian Mixed Model for Repeated Measures (MMRM) with a Hurdle-Gamma likelihood was used to evaluate the primary and secondary endpoints. The model was fit using PyMC,18 and uninformative priors scaled to the range of the data and on the log and logit scales for the appropriate parameters were applied. Study site, study arm, time and baseline area were included in the model as covariates for both the binomial and gamma components of the model.
TABLE 3 Baseline characteristics.
| SOC | LHACM | |
|---|---|---|
| Subjects, N (%) | 21 (29.2) | 50 (70.8) |
| Age, mean (SD) | 63.2 (10.9) | 61.9 (10.8) |
| Sex, N (%) | ||
| Male | 16 (76.2) | 42 (84.0) |
| Ethnicity, N (%) | ||
| Black | 3 (14.3) | 7 (14.0) |
| Native Hawaiian/Pacific Islander | 0 (0.0) | 2 (4.0) |
| White | 18 (85.7) | 41 (82.0) |
| Tobacco use, N (%) | ||
| Yes | 3 (14.3) | 6 (12.0) |
| Fitzpatrick scale, N (%) | ||
| Type I | 2 (9.5) | 10 (20.0) |
| Type II | 9 (42.9) | 19 (38.0) |
| Type III | 6 (28.6) | 15 (30.0) |
| Type IV | 2 (9.5) | 2 (4.0) |
| Type V | 2 (9.5) | 3 (6.0) |
| Type VI | 0 (0.0) | 1 (2.0) |
| Wagner grade | ||
| Grade 0 | 1 (4.8%) | 0 (0.0%) |
| Grade 1 | 13 (61.9%) | 24 (48.0%) |
| Grade 2 | 7 (33.3%) | 26 (52.0%) |
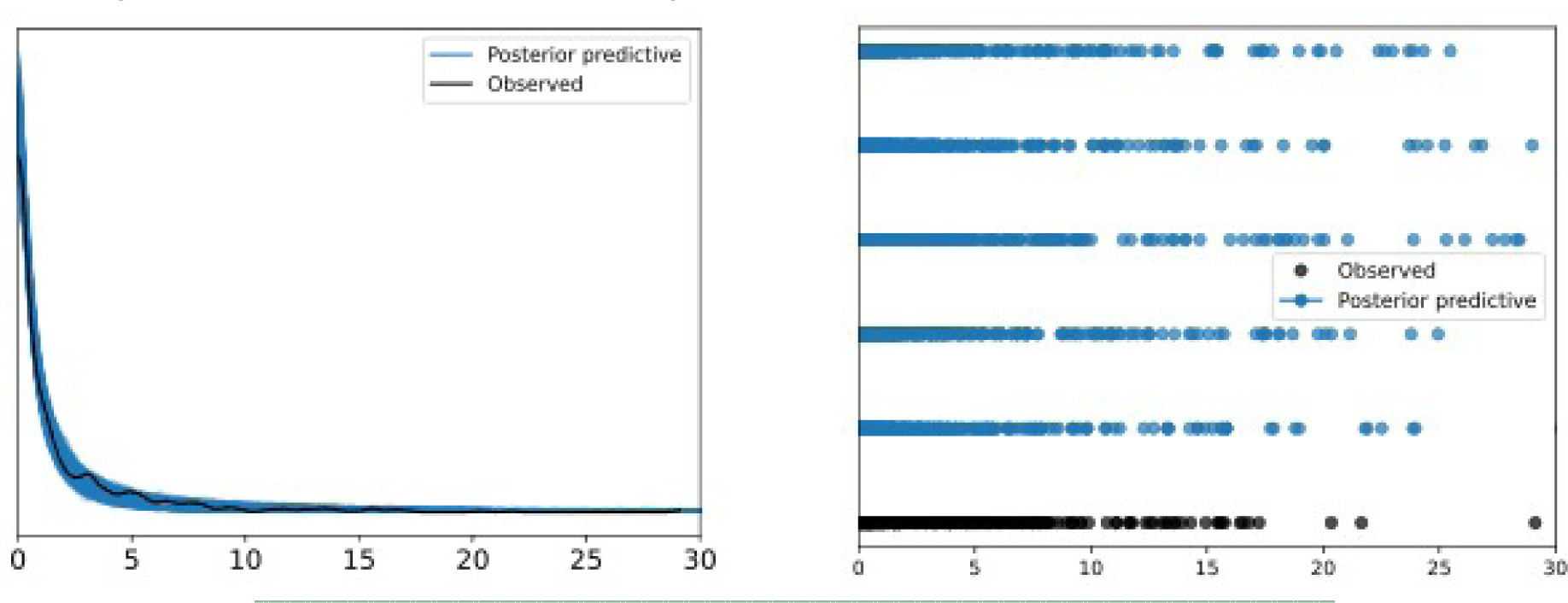
Model diagnostics were performed using a Markov Chain Monte Carlo (MCMC) parallel) plots, visual posterior predictive checks, and Leave-One-Out (LOO-CV). The MCMC parallel plots showed no divergences. The posterior predictive checks successfully reproduced the empirical distribution of the observed wound area, including the spike at zero and near zero, reflecting closed or nearly closed wounds, confirming the Hurdle Gamma distribution appropriateness, shown in Figure 1. LOO-CV demonstrated stable out-of-sample predictive accuracy, with 98% of timepoints falling within an acceptable Pareto K diagnostic threshold of equal to or less than 70%. The distribution of the Pareto K values is provided in the Statistical Appendix.
FIGURE 1 Posterior Predictive Checks, KDE (left) and Scatter (right).
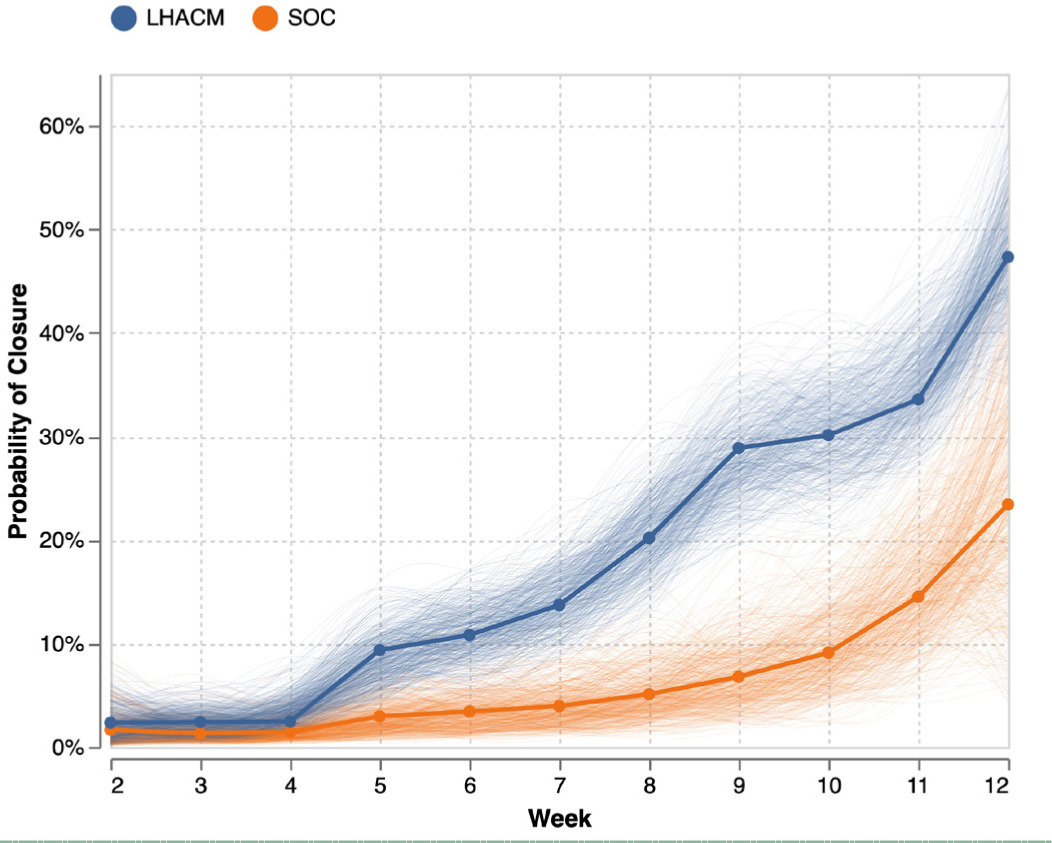
Group average marginal effects (G-AME) at week 12 were then calculated using the posterior and posterior predictive for the primary and secondary endpoints respectively, shown in Figure 2. The estimated probability of complete wound closure under SOC was 23% (6.5%–42%), compared with 47% (37%–59%) under the treatment arm This corresponds to an posterior absolute difference of 24% (4%–43%) and a risk ratio of 2.6 (0.8–5.0) in favor of LHACM. This suggests a posterior probability advantage of 98.5% for LHACM. Wounds managed with SOC demonstrated a mean PAR of 92% (81%–99%), whereas wounds in the LHACM arm showed a mean reduction of 73% (56%–89%), shown in Figure 3. The Bayesian survival plot in Figure 4 shows the probability of wound closure at each treatment visit.
FIGURE 2 Group average marginal effects: SOC Wound Closure (top left); Treatment Wound Closure (top right); Absolute Difference (bottom left); and Risk Ratio/Relative Risk (bottom right).
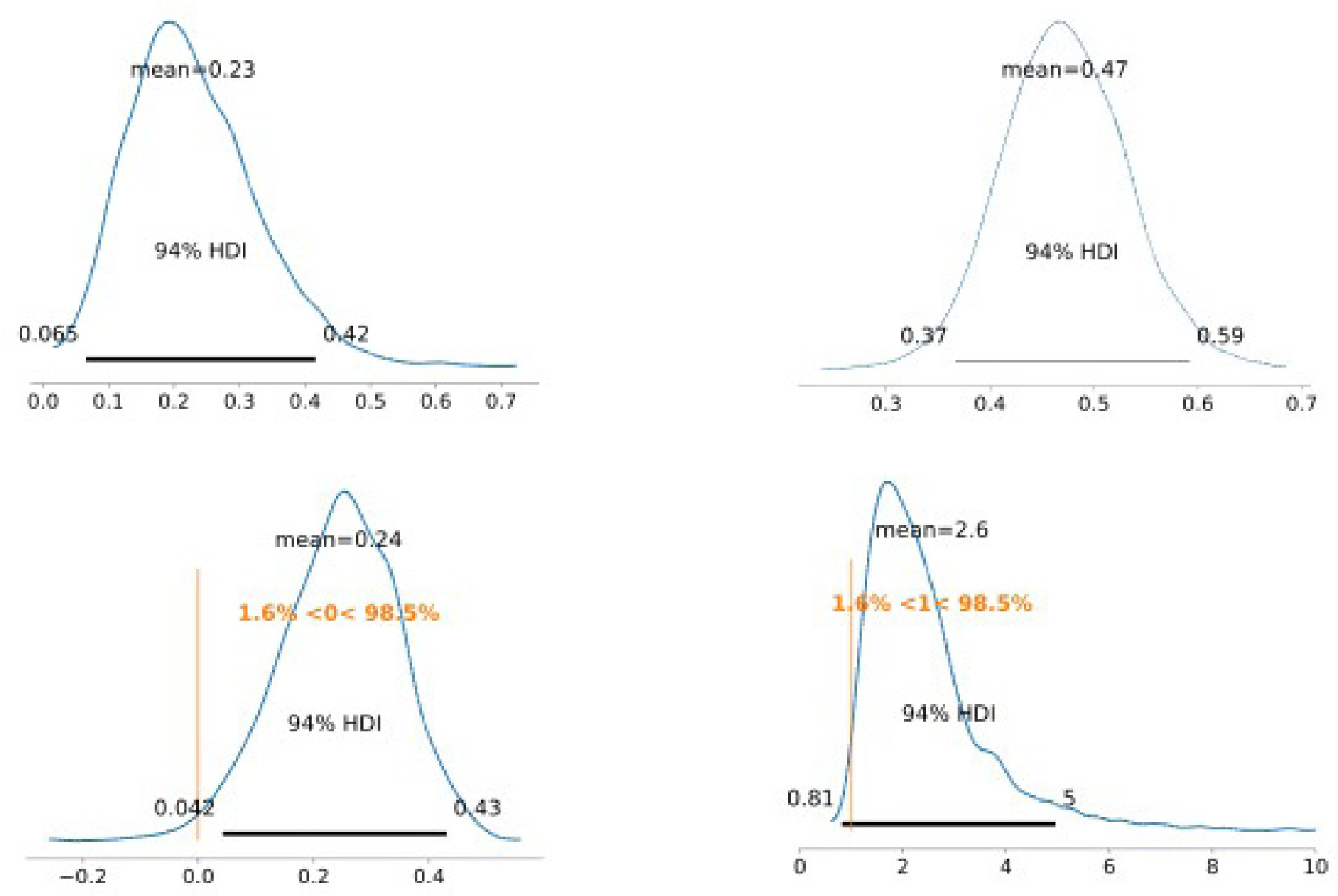
FIGURE 3 Group average marginal effects: SOC PAR (left); Treatment PAR (right).
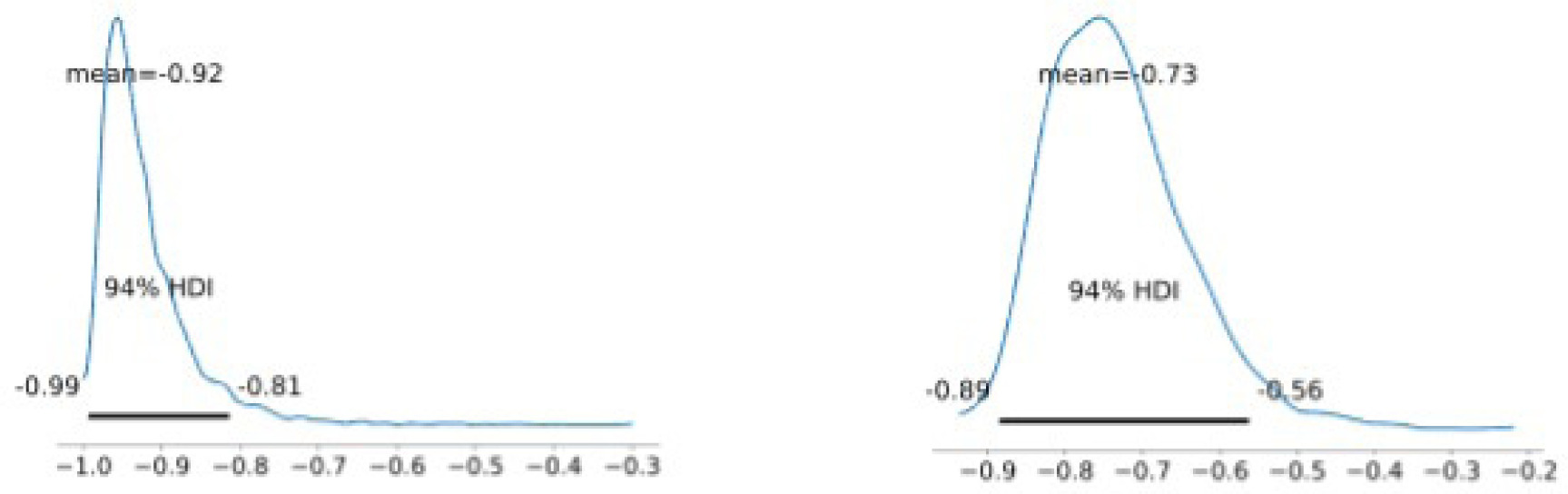
FIGURE 4 The Bayesian survival plot shows the probability of wound closure at each treatment visit.
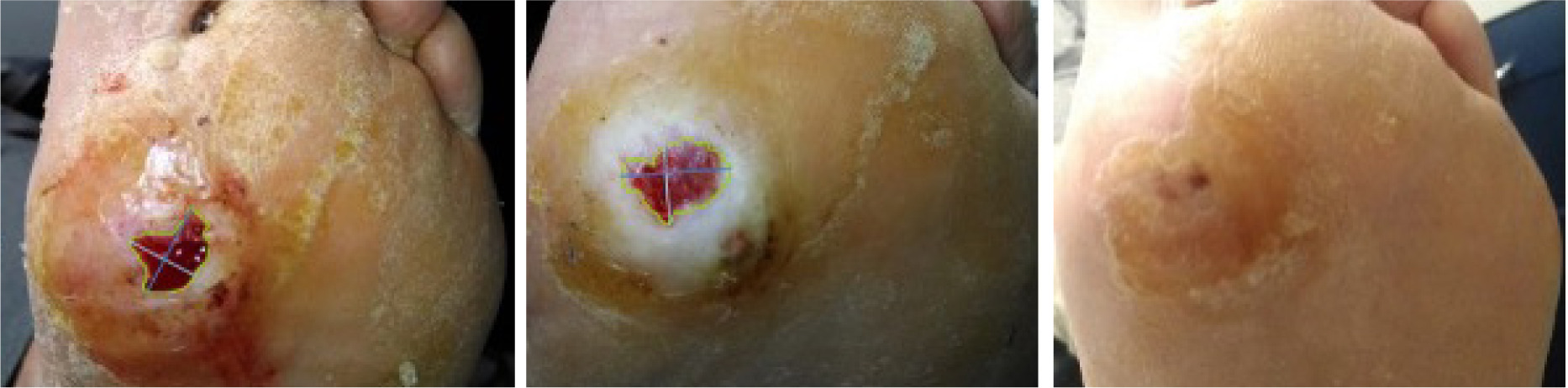
Sequential images shown in Figure 5 document the trajectory of wound healing from SV-1, TV-1, and HCV in a patient assigned to the LHACM with SOC treatment arm.
FIGURE 5 Digital images from SV-1, TV-1, and HCV (left to right), LHACM with SOC treatment arm Patients gave consent for publication of images.
Discussion
This interim analysis compared wound healing outcomes between SOC and LHACM. At week 12, the probability of complete wound closure was higher under LHACM (47%) than SOC (23%), with a 98.5% posterior probability that Treatment improved closure. Conversely, mean percent area reduction (PAR) favored SOC (92% vs. 73%), with a 97% posterior probability that SOC achieved greater area reduction.
The observed difference reflects the structure of the Bayesian hurdle-gamma model, which separately models the probability of closure and the conditional distribution of area reduction among non-closers. In the LHACM arm, a higher proportion of wounds reached closure by Week 12, leaving a smaller pool of resistant wounds with limited shrinkage and thus a lower mean PAR. By contrast, SOC patients were less likely to close, leaving more wounds in the '"partially healed'" state that continued to show large proportional reductions in size. Taken together, the closure and PAR results are consistent and complementary: LHACM accelerates full closure, whereas SOC patients more often remain in partial healing with ongoing area reduction.
These results are in line with the most recent DFU RCTs on dehydrated human amnion/chorion membrane (DHACM) and dehydrated human umbilical cord (DHUC) which had deltas of 20% and 22% respectively.19,20 The lower absolute healing rates in the LHACM and SOC arm reflect the more stringent screen failure design in this trial to eliminate fast healers. Both DHACM and DHUC are on the covered lists of the most recent LCDs.
Bayesian statistics are well recognized by the FDA and CMS. The FDA has a Guideance document entitled, 'Guidance for the Use of Bayesian Statistics in Medical Device Clinical Trials' from 2010 supporting the use of Bayesian statistics for regulatory approval studies. Additionally, the FDA and CMS have approved numerous devices and drugs using Bayesian statistics, including a Medtronic's Cardiac Defibrillator, Medtronic's Transcatheter Heart Valve for Intermediate Risk Patients, Neuropace's Neurostimulation for Epilepsy, Dexcom's G6 Continuous Glucose Monitor, Pediatric Cochlear Implants, Endovascular Aneurysm devices, and the drugs Benlysta, Jardiance, and Tradjenta. The use of Bayesian statistics in all the examples listed allowed for much earlier approval by FDA and CMS using predictive modeling. The studies later confirmed their interim Bayesian analysis. Hence, the Bayesian analysis of the LHACM data provides support for CMS adding LHACM to the covered list for DFUs, and this data will be confirmed with the full study analysis.
Conclusion
The interim analysis revealed that the placental membranes products trended to-ward superiority over SOC. Bayesian posterior estimates indicated 98.5% higher probabilities of wound closure and improved healing trajectories in the treatment group. These interim data provide early evidence of clinical benefit, subject to confirmation with full trial completion.
An interim analysis was conducted after the 71st participant completed randomization. Interim study success was defined as a posterior probability of at least 0.90 that the treatment effect, expressed in absolute or relative terms, exceeded zero or one, respectively. The posterior probability that LHACM was superior to standard of care in both measures was 0.985. Accordingly, the criterion was met, and therefore success declared.