Introduction
Hard-to-heal (chronic) wounds are a significant and growing public health concern, particularly in aging and medically complex populations. Among these, pressure ulcers (PUs)—also known as pressure injuries—represent one of the most prevalent and costly wound types. Globally, the incidence of PUs more than doubled between 1990 and 2021, increasing from 1.1 million to 2.5 million cases annually, with disability-adjusted life years (DALYs) rising proportionally during the same period.1 In the US, the burden is particularly high, with an increasing number of Medicare beneficiaries affected by PUs over time.2 Medicare spending on chronic wounds—including PUs—was estimated at $22.5 to $67 billion in 2019, with a significant proportion attributable to post-acute and home care settings.2
PUs are localized injuries caused by sustained pressure and shear, typically over bony prominences, and are often exacerbated by immobility, perfusion deficits, or incontinence. Healing of these wounds is frequently delayed by chronic inflammation, infection, and systemic comorbidities. Complete closure of stage III or IV PUs often requires many months, and recurrence rates remain high.3 Unhealed PUs are associated with a significantly increased risk of hospitalization and mortality, primarily due to sepsis and other systemic complications.4,5
In response to the therapeutic challenges posed by chronic wounds, clinicians have increasingly turned to advanced therapies, including cellular, acellular, and matrix-like products (CAMPs). CAMPs represent an evolving class of biologically active wound therapies, encompassing placental-derived matrices, synthetic scaffolds, and other constructs designed to modulate the wound environment by supporting extracellular matrix production, promoting angiogenesis, and mitigating inflammatory pathways.6 A 2023 consensus document formally defined CAMPs as a broad category of materials aimed at facilitating tissue repair and regeneration.7
Clinical trials and real-world evidence support the efficacy of CAMPs, particularly in diabetic and venous ulcers, where they have been shown to reduce healing time, major amputations, and use of hospital resources when used according to evidence-based protocols.8,9 A large Medicare cohort study involving more than 900,000 diabetic ulcer cases found that early use of skin substitutes reduced amputations and emergency visits compared to delayed or no use.8 While PUs remain under-represented in such studies, available evidence has shown promising results: CAMPs, with and without negative pressure wound therapy (NPWT), have improved healing outcomes in PUs, including full-thickness wounds.3,10,11 These findings highlight the potential relevance of CAMPs in PU management.
In addition to the inherent challenges of treating chronic wounds, access to advanced wound care remains a critical barrier for many patients, especially those in rural or mobility-limited settings. For example, rural Americans with diabetic foot ulcers (DFUs) face a 50% higher risk of major amputation compared to their urban counterparts, due to factors such as limited access to specialists and fragmented communication between providers.12,13 These geographic and functional barriers disproportionately affect patients in post-acute care—particularly those who are bedbound, wheelchair-dependent, or otherwise mobility-limited. Transportation challenges, limited local availability of specialized wound care, and caregiver burden exacerbate disparities in access to timely, evidence-based treatments.
In this context, mobile wound care—delivered in the patient’s home, skilled nursing facility, or assisted living environment—has emerged as a promising strategy to overcome access-related delays and provide consistent care continuity.14,15
Given these realities, evaluating CAMPs in the post-acute environment—especially among populations vulnerable to access constraints—is critical. This study aims to assess the real-world impact of CAMPs on healing trajectories and patient outcomes in PU patients treated by a large, multi-provider mobile wound care practice in post-acute settings. We hypothesized that the use of CAMPs would significantly improve healing rates compared to standard of care (SOC) among patients with PUs treated in these settings. By focusing on mobile and decentralized care models, the analysis seeks to illuminate opportunities for expanding access and improving outcomes through targeted deployment of regenerative wound technologies.
Materials and methods
Data source and study setting
All data were extracted from the LiftOff Registry maintained by Open Wounds Research. The LiftOff Registry is a centralized analytics environment designed to support real-time surveillance of PU prevalence, severity, and healing outcomes across post-acute care settings. The LiftOff Registry aggregates de-identified wound-level and patient-level data from a national network of participating long-term acute care hospitals (LTACHs), skilled nursing facilities (SNFs), and related sub-acute institutions. As of the most recent reporting period, the registry encompasses more than 18,000 patients and 23,000 PUs, representing one of the largest real-world datasets of its kind.
Data are extracted directly from partner electronic medical record (EMR) systems, harmonized through automated preprocessing pipelines, and standardized according to the LiftOff data model. This model captures detailed wound characteristics (location, stage, size, and depth), patient demographics, comorbidities, and care processes. Wounds are uniquely identified and linked longitudinally to enable tracking of healing progression, stage transitions, and outcomes over time.
All data are fully de-identified before analysis and stored within a secure, HIPAA-compliant environment. The registry operates under a centralized governance framework, which includes oversight for data integrity, ethical use, and authorized secondary analysis for research and quality improvement.
The study dataset included adult patients (≥18 years) treated in the period from January 2022 to January 2025 with at least one documented PU and sufficient follow-up to assess healing status. Wounds were excluded if key baseline variables were missing. Healing was defined as complete re-epithelialization or complete closure, as documented in the clinical record by the treating provider. Wound-level identifiers enabled longitudinal tracking to verify closure events across multiple encounters. The study was conducted in compliance with applicable HIPAA regulations. Use of the registry data for secondary research and publication was approved under the registry’s governance and data-use framework; formal Institutional Review Board (IRB) review was deemed unnecessary due to the retrospective analysis of de-identified data.
Statistical analysis
This observational comparative effectiveness study evaluated wound-healing outcomes among patients receiving either SOC alone or SOC combined with CAMPs. Bayesian propensity score methods were applied to reduce confounding and improve balance between groups before estimating treatment effects on healing outcomes. All Bayesian models were implemented in Python 3.12 using the probabilistic programming library PyMC (version 5.23.0), with the Bayesian Model-Building Interface (version 0.15.0) serving as the modeling interface and ArviZ (version 0.21.0) used for posterior diagnostics and visualization.
Bayesian methods were selected to provide a coherent probabilistic framework for estimating treatment effects and for propagating uncertainty across both the matching and outcome stages. Bayesian inference generates full posterior distributions that quantify uncertainty in treatment assignment and allow this uncertainty to be carried forward into causal effect estimation.16-20 Several prior applications have demonstrated the advantages of Bayesian propensity score modeling for observational causal inference.20,21 The Bayesian framework also allows flexible hierarchical modeling of clustered data, partial pooling, and robust estimation under conditions of limited overlap or small sample size. Together, these features make Bayesian methods particularly appropriate for real-world wound data, where treatment assignment is nonrandom, empirical wound-area distributions are mixtures, and patient and wound heterogeneity is expected.
All regression coefficients were assigned weakly informative priors (Normal [0, 1]), and the intercept was assigned a Normal (0, 1.5) prior. Four Markov chain Monte Carlo (MCMC) chains were run with 1,000 warm-up and 1,000 posterior draws per chain using the No-U-Turn Sampler. Posterior mean propensity scores were calculated for each wound and used for 1:1 nearest-neighbor matching between SOC and treatment groups.
The matched dataset contained 375 wounds (190 treatment; 185 SOC). Post-matching, covariate balance was verified across age, baseline wound area, and presence of diabetes, indicating that the Bayesian matching procedure achieved satisfactory overlap of covariate distributions between groups.
Within the matched cohort, the probability of wound healing was modeled using a Bayesian logistic regression, with study arm and baseline wound stage specified as predictors. Posterior predictions were summarized as posterior means with 95% credible intervals (CrI) for each arm and wound stage.
Results
Before matching, the SOC cohort included 9,356 patients (14,740 wounds; 33.2% healed), while the treatment cohort included 136 patients (190 wounds; 49.5% healed). After Bayesian propensity score matching, 375 wounds were retained (190 treatment; 185 SOC). Post-matching, baseline characteristics were well-balanced between study arms across demographic and clinical variables, including age, diabetes prevalence, and baseline wound area. The stage distribution was also comparable, with most wounds classified as stage 3 or 4. These findings confirm that the Bayesian matching procedure achieved satisfactory covariate balance between groups (Table 1).
The usage patterns of CAMPs in the treatment group were heterogenous, with an average of 17 weeks of SOC treatment before the first CAMPs application. An average of 6.23 CAMPs products were applied to the healed wounds in the treatment group, with an average of 6.45 applications for the non-healed wounds.
The primary analysis estimated the average treatment effect (ATE) of CAMPs on the likelihood of wound healing— defined as complete re-epithelialization or closure documented in the clinical record—using a Bayesian logistic regression model with study arm and baseline wound stage as predictors. Posterior draws from the model yielded a posterior mean ATE of 0.16 (95% CrI, 0.15 –0.18), indicating that treatment with CAMPs increased the probability of healing by approximately 16 percentage points relative to SOC (Table 2).
TABLE 1 Baseline characteristics of the matched cohorts (SOC (Control) vs CAMPs+SOC (Treatment)
| Matched control | Matched treatment | |
|---|---|---|
| Wounds | ||
| Wounds | 179 | 190 |
| Sex | 89 (49.7%) | 94 (49.5%) |
| Age (mean) | 65.4 | 66.7 |
| Diabetic | 30.0 (16.8%) | 31.0 (16.3%) |
| Starting area (median) | 9.0 | 9.5 |
| Stages | ||
| Deep tissue injury | 4 (2.2%) | 9 (4.7%) |
| Stage 2 | 1 (0.6%) | 7 (3.7%) |
| Stage 3 | 38 (21.2%) | 41 (21.6%) |
| Stage 4 | 119 (66.5%) | 118 (62.1%) |
| Unstageable | 17 (9.5%) | 15 7.9%) |
TABLE 2 Posterior mean average treatment effect and 95% credible intervals by wound stage and treatment arm
| Matched control | Matched treatment | |
|---|---|---|
| Overall | 32.9% (24.1–41.7%) | 49.2% (38.6–59.4%) |
| Stage | ||
| Deep tissue injury | 38.4% (21.6–55.4%) | 54.6% (37.4–72.8%) |
| Stage 2 | 37.4% (12.0–61.9%) | 52.8% (25.4–76.8%) |
| Stage 3 | 28.7% (18.7–38.2%) | 44.1% (33.0–55.5%) |
| Stage 4 | 34.7% (27.3–42.0%) | 51.2% (43.2–58.5%) |
| Unstageable | 28.1% (15.2–43.6%) | 43.0% (26.0–60.0) |
These results represent an approximate 50% relative increase in healing probability compared with SOC alone. The 95% credible interval for the posterior ATE excluded zero, demonstrating that the observed treatment effect is statistically credible within the Bayesian framework. A similar effect was observed across the spectrum of PU stages, including severe Stage IV ulcers and deep tissue pressure injuries (DTPI).
The posterior distributions of the estimated treatment effects demonstrated clear separation from zero, with the CAMPs arm consistently centered in the positive domain (Figure 1). The 95 percent credible intervals were narrow and excluded zero, confirming a statistically credible and clinically meaningful improvement in healing. Together, these findings demonstrate a robust positive association between CAMPs use and improved wound closure in post-acute care settings.
Discussion
These results indicate that the use of CAMPs was advantageous to PU healing in the post-acute setting. The posterior mean ATE of 0.16 (95% CrI, 0.15 –0.18), demonstrates a statistically credible and clinically meaningful benefit, corresponding to approximately a 16-percentage-point improvement—or a 50% increase—in the probability of complete re-epithelialization compared with SOC. This finding is consistent with results of other studies involving PUs or chronic wounds more generally.3,8-11 The analysis suggests that CAMPs are a valuable adjunct in the treatment of PUs in post-acute care, helping to reduce the burden of these wounds on patients in an environment with increased risk. Of particular interest, the effect of CAMPs was positive across the full spectrum of baseline PU stages, including stage IV and deep tissue pressure injury.
FIGURE 1 Posterior distribution of the average treatment effect of CAMPs vs SOC on the likelihood of complete re-epithelialization..
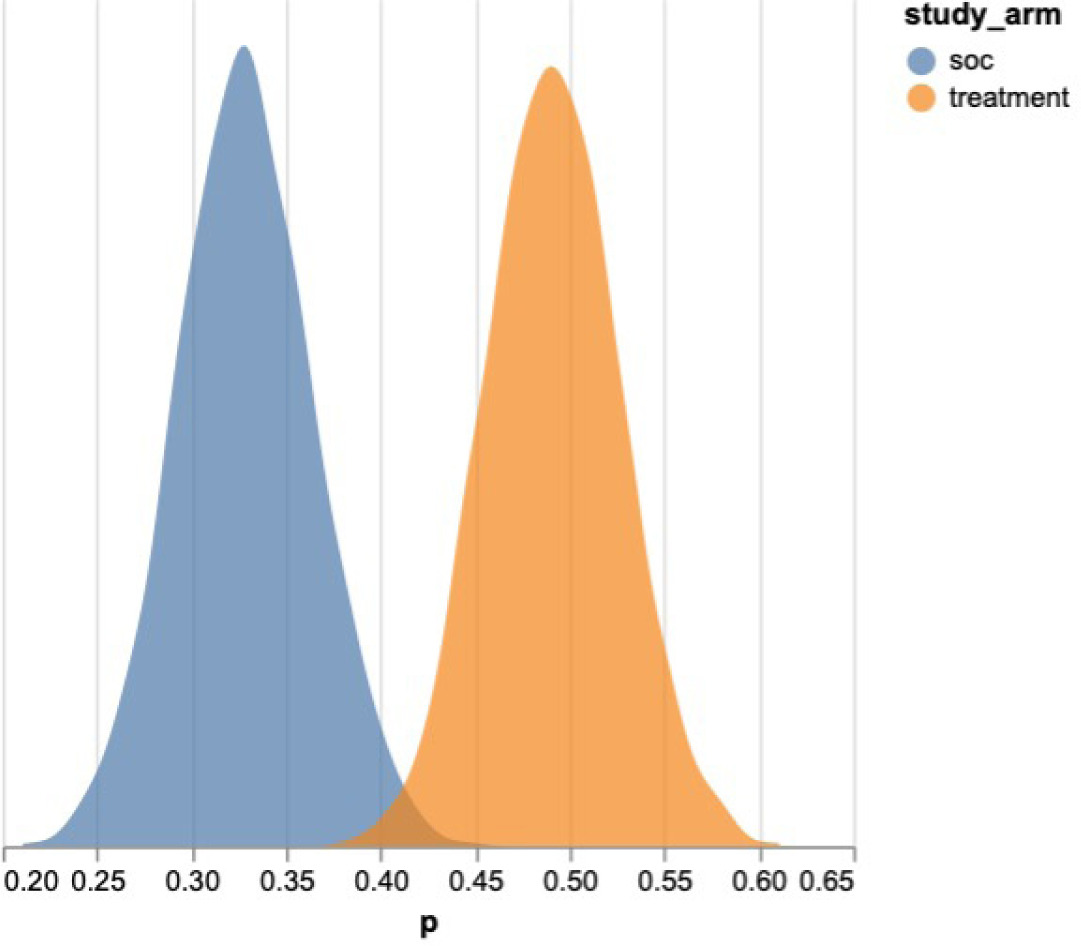
The analysis did not attempt to differentiate between CAMPs by type or brand; rather, it considered all CAMPs applications in the registry. Areas for further research include comparative analysis of specific CAMPs to identify potential differences in efficacy, cost-effectiveness, and optimal use scenarios.
Previous real-world studies of CAMPs have indicated that these products are most beneficial when applied early (after approximately 4 weeks of non-healing) and administered continuously at 1- or 2-week intervals, as recommended by product manufacturers.8 The usage patterns observed in this dataset were heterogeneous, with an average first application after 17 weeks—substantially later than the recommended 4-week window. The scope of the dataset was not sufficient to determine whether earlier applications would have produced greater benefit in this population. This question remains an important area for further investigation.
These findings suggest that integrating CAMPs into standard post-acute wound management protocols could shorten healing trajectories and reduce downstream complications such as infection, hospitalization, and caregiver burden. Given the complexity and chronicity of PUs observed in this population, CAMPs appear particularly well-suited for difficult-to-heal or non-healing wounds in medically complex patients commonly encountered in post-acute settings. Accelerated healing in these contexts may also reduce long-term healthcare expenditures and resource use associated with chronic wound care.
Strengths and limitations
It is important to note that real-world studies such as this one lack the strict controls of randomized controlled trials (RCTs) and therefore may reflect a broader range of patient demographics, wound types, and pre-treatment durations. Methodological limitations include missing or incomplete data, variability in wound measurement techniques and CAMPs usage patterns, potential selection bias by individual clinicians, and unmeasured confounding variables. Although the Bayesian-matched cohort approach mitigates several of these limitations by improving covariate balance and propagating uncertainty through the model, residual bias cannot be entirely eliminated.
In terms of generalizability, the study population likely reflects the heterogeneity typical of post-acute wound care, including patients with large, long-duration wounds and multiple comorbidities. Conversely, limited RCT data are available on the use of CAMPs to treat PUs, and RCT populations often fail to capture the complexity of wounds encountered in real-world settings.22 For example, the mean baseline wound area in this study exceeded 23cm2 in both groups—substantially larger than wound sizes typically reported in chronic wound RCTs. As such, these real-world findings may more accurately represent the outcomes achievable in everyday clinical practice, particularly within post-acute environments.
No safety or adverse event data were captured in this dataset. However, no reports of CAMPs-related complications were identified in the available clinical documentation, consistent with prior literature describing favorable tolerability profiles for these products.
Conclusion
To our knowledge, this study is the first to evaluate the impact of CAMPs on healing outcomes in a real-world population of patients with PUs treated by mobile wound providers in the post-acute setting. The results of this retrospective matched-cohort analysis indicate that CAMPs were beneficial to healing, with effects consistent across the full spectrum of baseline PU stages, including stage IV and deep tissue pressure injury. Earlier initiation of CAMPs might yield even greater benefits.
These findings suggest that integrating CAMPs into standard post-acute wound management protocols could shorten healing trajectories, reduce complications, and improve patient and caregiver outcomes. Broader adoption of CAMPs within decentralized wound care programs—particularly where access to advanced therapies is limited— may represent an important step toward improving equity and outcomes in chronic wound management. This study provides a foundation for future prospective evaluations to confirm and extend these real-world findings.