Introduction
Wound healing is a complex, multi-phase biological process that proceeds in a defined sequence as the body responds to injury and the surrounding environment.1 When this process is disrupted for more than four weeks, the wound is classified as chronic and non-healing. Venous leg ulcers (VLUs), which result from chronic venous insufficiency (CVI), are among the most difficult chronic wounds to treat.2 VLUs represent the most common chronic ulcer type, with a prevalence of 1.5–3 cases per 1,000 individuals and accounting for 50–70% of lower-leg ulcers.3,4
These wounds contribute to significant morbidity and healthcare expenditure, with an estimated annual cost approaching $15 billion.3,4-7 Epidemiological data also indicate higher mortality and poorer outcomes among patients with VLUs.8
Several risk factors for VLUs have been identified, many of which are non-modifiable. Advanced age is a major determinant, with prevalence rising to 1.5% among individuals older than 65 years.6,9 Other contributing factors include prior thromboembolic events, musculoskeletal disorders, obesity, female sex, and genetic predisposition.3,4,10
The etiology of VLUs is multifactorial involving structural and functional abnormalities of the venous system and failure of the calf-muscle pump, leading to venous hypertension and ulcer formation.11 Mechanistic contributors include immune dysregulation, alterations in extracellular-matrix remodeling, cytokine imbalance, and aberrant connexin-43.12
A deeper understanding of these biological pathways may facilitate the development of targeted therapies to improve venous-ulcer healing.13-16
Recurrence of VLUs can be caused by various factors such as advanced age, larger wound area, obesity, co-existing venous disease and nutritional deficiencies.16,17 For each 1cm2 in ulcer size, the risk of treatment failure increases by 11%.17 The standard of care (SOC) for VLUs is compression therapy, which enhances venous return, reduces edema, and optimizes tissue perfusion to support repair.11 However, compliance remains a challenge due to discomfort, pain, or poor patient understanding of its benefits, underscoring the need for alternative or adjunctive treatments.2,18-20
Cellular and tissue-based products (CAMPs) and skin grafting have been increasingly incorporated into VLU management.21-23 Randomized trials demonstrate that CAMPs significantly enhance wound closure compared with compression alone (SOC), with dehydrated amnion-chorion membranes showing improved healing within defined treatment windows, with dehydrated amnion-chorion membranes showing improved healing within defined treatment windows.24 However, these studies supported the beneficial role of CAMPs in VLU healing.21-23,25-28 Apligraf (Organogenesis, USA), an FDA-approved bilayered living skin construct, achieved 47% healing at six months versus 19% with compression alone.26 CAMPs provide both cellular and matrix support while delivering cytokines and growth factors that promote tissue regeneration.23,29,30
Placenta-derived tissues—including amniotic membrane, amniotic fluid, umbilical cord, or composite placental tissue—constitute a category of CAMPs with potent regenerative properties.23,27-29 These materials promote angiogenesis, modulate inflammation, and limit fibrotic response, thereby accelerating repair.29 Clinical studies have reported 70% healing at 12 weeks with amniotic-based allografts compared with 30% using SOC alone.25 Although prior trials have been limited in scale, meta-analyses consistently support improved outcomes among patients treated with amniotic-membrane allografts.22,27,28 Larger randomized studies remain warranted to further validate these findings.
AmnioBurgeon (OneBiotech, USA) is a resorbable, chorion-free human amnion allograft derived from donated human birth tissue, which has been approved for the management of VLUs. Rich in collagen, growth factors, and extracellular-matrix proteins, AmnioBurgeon supports the biological processes essential to wound repair and has demonstrated favorable outcomes in patients with diabetic foot ulcers (DFUs).
In this retrospective study, we have analyzed the effect of AmnioBurgeon treatment on VLUs.
Patients and methods
Data source
The medical charts reviewed in this study spanned the period from March 2025 to September 2025. All treatment records for patients meeting eligibility criteria who received applications of AmnioBurgeon were extracted from the database and de-identified. Atlas 360 electronic medical records were reviewed for the patient’s demographic information, clinical history, and laboratory results. Patients’ characteristics such as age, gender, weight, comorbidities, and laboratory values such as Ankle-Brachial Index (ABI) were taken. Additional information includes: number of AmnioBurgeon applications, treatment duration, and wound-related examination. Wound area (in cm2) was assessed prior to each treatment wound duration, location, complexity, and signs and symptoms of infection were also documented.
Ethical approval
For this retrospective study, the Institutional Review Board (IRB), UNIVO, has determined that the study is exempt from IRB oversight requirements under 45 CFR 46.104(d) and under category 4 (#STU25090176). The IRB has approved the request for a waiver of consent and a waiver of authorization for this study.
Study subjects
The study included medical charts of patients who were treated at United WoundCare Institute in Naperville, IL. Those patients were selected based on eligibility criteria, which are summarized in Table 1.
Outcome and follow-up
The primary goal of this study was to evaluate the change in wound area after AmnioBurgeon treatment for VLUs.
TABLE 1 Eligibility criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Complete wound closure was defined as 100% re-epithelialization, as determined by the treating physician. Partial reduction was defined as ≥50% reduction in wound area. No response was defined as <25% reduction in the initial two weeks of treatment or an increase in size. Percentage of wound area reduction, time to reduction, complete wound closure, and number of AmnioBurgeon applications that are required for reduction were also determined. Infections during treatment were assessed by signs and symptoms without the requirement of culture confirmation.
Statistical analyses
Statistical analyses were conducted using Python (version 3.9.21) and the following packages: Pandas (2.1.4), Numpy (1.26.4), Statsmodels (0.14.0), SciPy (1.11.4), Lifelines (0.28.0), Matplotlib (3.8.2), Seaborn (0.13.0), and the builtin datetime module. Demographics and clinical variables were summarized. Descriptive summaries of continuous variables included the mean, standard deviation, median, and range. Categorical variables were summarized in frequencies and percentages.
A mixed-effects linear regression model accounting for repeated measures was performed to assess significance (p<0.05) in the change of wound area before and after treatment, and between applications. Baseline wound size areas were placed into two groups (<10 cm2, and 10 to <24.99 cm2). Wounds were separated based on age into 2 groups: <180 days or ≥180 days. Chi-squared test was performed to compare the proportion of wounds achieving ≥50% area reduction after 28 days of treatment between groups. Kaplan-Meier analysis was performed to compare the time to ≥50% wound area reduction between groups. Recurrent wounds were excluded from analysis to avoid over-representation.
Results
Patients’ demographics and wound characteristics
In this study, 47 VLUs in 38 patients were treated with AmnioBurgeon after failing to heal with SOC therapy. The median age of the patients was 79.6 (range 48–100), and 47% were female (Table 2). At baseline, the median wound area was 8.8 cm2 (range 1.2–20.3). The median wound duration prior to CAMP treatment was 180 days, and most wounds have their fat layers exposed (~39%). Wounds were treated with CAMP on average every 7.4±1.1 days, as literature suggests, reflecting both the biologic needs of wound healing and the realities of patient care delivery.31,32
TABLE 2 Patient demographics and wound characteristics
| Number of patients | 38 |
|---|---|
| Age, years (Mean±SD, Median, Min, Max) | 79.03 ±10.8, 79.6, 48.8, 100.24 |
| Female (N, %) | 18 (47%) |
| Number of wounds | 47 |
| Wound locations | |
| Lower leg | 35 |
| Feet | 12 |
| Stage/severity | |
| Fat layer exposed | 15 |
| Breakdown of Skin | 6 |
| Unspecified severity | 25 |
| Muscle involvement without evidence of necrosis | 1 |
| Without bone or tendon exposure | 0 |
| Without evidence of necrosis | 0 |
| Wound size at baseline (cm2) (Mean±SD, Median, Min, Max) | 9.4±5.1, 8.8, 1.2, 20.3 |
| Wound Age (Days) Median | 180 |
| Time from First Visit to CAMPs Treatment, (Days) (Mean±SD, Median) | 16.8±31.5, 126.0 |
The median number of CAMP applications per wound was 6 (range 2-12). The median duration of the CAMP treatment course was 35 days (7-96).
Wound healing efficacy outcome
AmnioBurgeon treatment resulted in an overall favorable outcome in VLUs that failed SOC treatment (Table 3). Of the 47 wounds, 37 (46%) wounds achieved more than ≥50% reduction in wound area. The median time to ≥50% reduction was 14 days (range 7, 49) and required a median of 6 (range 2, 12) CAMP applications. At least 50% wound area reduction after 28 days of treatment is a strong predictor of potential complete wound closure, which was observed in 20 (42%) wounds. This result showed a substantial early response to the treatment. AmnioBurgeon treatment resulted in complete wound closure in 6% of wounds. The median time to complete wound closure was 42 days (range 14-72) and required a median of 4.5 applications. No response (<25% reduction or increased size) was noted in 14 (29%) wounds.
TABLE 3 Wound healing treatment outcomes
| Completely Healed Wound (%) | 3 (6%) |
|---|---|
| Complete healing time (days) (Mean±SD, Median, Min, Max) | 38.8±19.6, 42.0, 14.0, 72.0 |
| Number of CAMPs Applications for Completely Healed Wound (Mean±SD, Median, Min, Max) | 4.2±1.6, 4.5, 2.0, 6.0 |
| Partial Healed Wound (>=50%) | 22 (46%) |
| Partial Healed time (days) (Mean±SD, Median, Min, Max) | 17.6±12.4, 14.0, 7, 49 |
| >=50% reduction by day 28 (%) | 20 (42%) |
| Number of CAMP Applications for Partial Healed Wound (Mean±SD, Median, Min, Max) | 6.4±2.9, 6.0, 2, 12 |
| No Response (<25% reduction or increased size) (%) | 14 (29%) |
| Duration of CAMPs Applications (Mean±SD, Median, Min, Max) | 39.1±23.5, 35.0, 7, 96 |
| Number of CAMPs Applications (Mean±SD, Median, Min, Max) | 6.2±3.0, 6.0, 2, 12 |
| Time until Max PAR (Mean±SD, Median, Min, Max) | 39.10, 23.53, 35, 7, 96 |
| Duration between CAMPs Applications (days) (Mean±SD, Median, Min, Max) | 7.4±1.1, 7.0, 6, 14 |
| ABI (n, Mean, Median, Min, Max) | 47, 0.887234, 0.86, 0.6, 1.32 |
| Infection (n, %) | 13 (34%) |
Baseline wound size
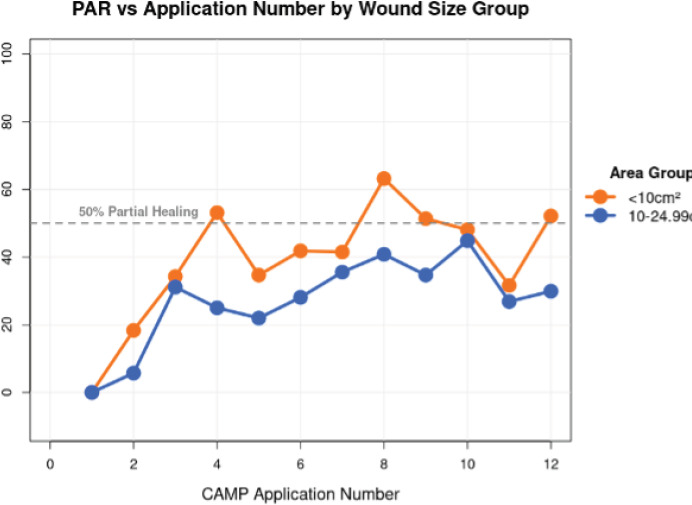
Baseline wound size impacted the percent area reduction with the number of CAMP treatment applications (Figure 1). The PAR effect of more CAMP applications for wound sizes <10cm2 was more pronounced than for wounds between 10 and 25 cm2.
FIGURE 1 In a mixed-effects linear regression model accounting for repeated measures within wounds, each additional Cellular, Acellular, Matrix-like Product (CAMPs) application was associated with a significant increase in percent area reduction for smaller wounds (PAR) (p=0.068).
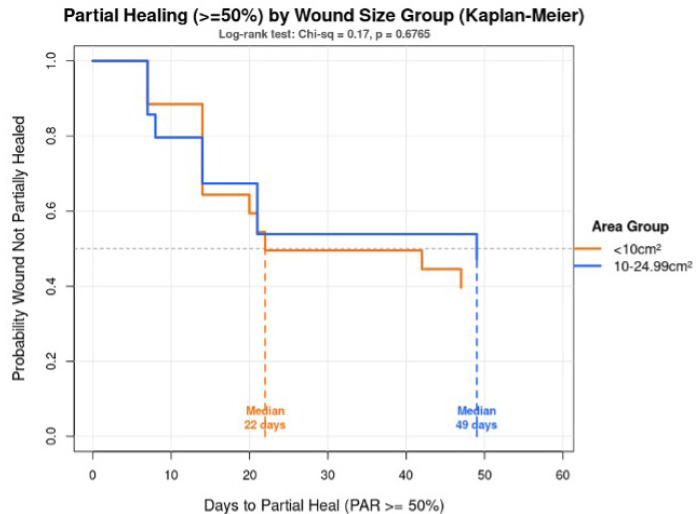
Additionally, baseline wound size was shown to affect the likelihood of achieving ≥50% reduction in wound area after treatment (Figure 2). Kaplan-Meier analysis with log-rank test was conducted to show wounds <10 cm2, 53.8% achieved partial healing at 22 days, while 42.9% of wounds sized between 10-24.99 cm2 achieved partial healing in 49 days. Findings showed that smaller wounds trended towards faster healing, but the difference was not statistically significant (p=0.68).
FIGURE 2 Kaplan-Meier analysis across wound size groups to time of partial healing. Description: Time to partial healing (≥50% PAR) by wound size. Survival curves showing time until wounds achieve ≥50% area reduction, comparing wounds that are (<10cm2) vs (10-24.99cm2). Key findings: Median time to partial healing <10cm2 (53.8%) wounds in 22 days, while 10-24.99cm2 (42.9%) wounds in 49 days. Statistical test: Log-rank test: x2=0.17, p=068 (not significant). Conclusion: Smaller wounds exhibited a trend toward faster healing (22 vs 49 median days), but the difference was NOT statistically significant. The sample size may be too small to detect a true difference, or there is substantial overlap in healing times between the two groups. .
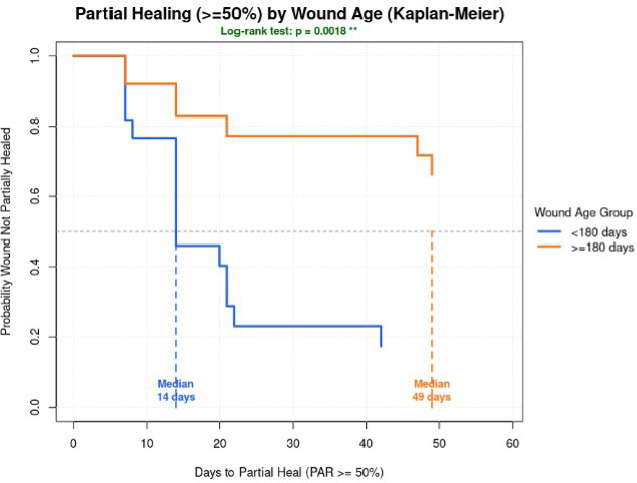
Wound age was found to significantly affect the response to AmnioBurgeon treatment (Figure 3). Wounds aged <180 days (n=22) reached partial reduction by day 14 in 72.7% (n=16) wounds compared to only 28% (n=7) in wounds with age >= 180 days (n=25). Wounds aged <180 days demonstrated a statistically significantly shorter time to achieving partial healing (log-rank p=0.0018).
FIGURE 3 Kaplan-Meier analysis with a log-rank test demonstrated a statistically significant difference in time to partial healing between wounds aged <180 days and ≥180 days (log-rank p = 0.0018), with younger wounds achieving partial healing more rapidly.
Discussion
VLUs are a debilitating healthcare problem with significant morbidity and costs.5 The leading underlying pathology for VLUs is chronic venous insufficiency, and this forms the basis and emphasizes the necessity of compression therapy in the treatment of VLUs.33 SOC for VLUs, including previously mentioned compression therapy, dressing, and others. However, the healing rate is still unsatisfactory. Several factors have been identified as contributing to treatment failure. This includes advanced age, which is associated with less mobility; a factor known to aggravate the underlying pathology, while other risk factors include wound age.
The use of CAMPs in the treatment of chronic wounds, including VLUs, is growing. Skin grafting is a primary therapy for wounds larger than 25cm2. Several studies have demonstrated higher closure rates with amniotic-based CAMPs.24 Despite the results being promising, larger-scale studies are needed. In our study, we analyzed retrospective data of patients treated with AmnioBurgeon for their hard-to-heal VLUs that were unsuccessful with SOC alone. Our results were encouraging despite several challenging factors in the baseline patient population, such as advanced age (mean 79.6), patient compliance, and other ongoing comorbidities.
In this retrospective analysis, we studied the effect of AmnioBurgeon on VLU healing. AmnioBurgeon is a resorbable, chorion-free human amnion allograft derived from donated human birth tissue, which has been approved for patients with VLU. We measured the effect of AmnioBurgeon across 47 wounds that failed SOC treatment. AmnioBurgeon was found to result in complete wound healing in 6% of wounds and wound size reduction by 50% or more at 4 weeks in 42% of patients. This is a strong predictor of complete healing at 12 weeks,34-36 and it serves as a valid endpoint in chronic wound management. Performing the same tests, we found a significant difference in time to partial healing between wounds aged less than 180 days and those with more than 180 days. This may further suggest that wounds that have been ongoing for more than 6 months are already very resistant and associated with poorer outcomes.37
Study limitations
This study has several limitations. It was a single-center, retrospective analysis with a relatively small sample size, which may limit the generalizability of the findings. The study design inherently lacked randomized controls, making it difficult to account for potential confounding variables or to establish causality. Additionally, variability in patient compliance with compression therapy and follow-up visits may have influenced healing outcomes. The short duration of observation also restricts the assessment of long-term wound closure rates and recurrence. Future larger, multicenter, and prospective studies with extended follow-up and standardized treatment protocols are needed to validate these findings.
Conclusion
Given the complexity and systemic nature of VLUs, they remain among the most challenging chronic wounds to treat. Despite these challenges, AmnioBurgeon has demonstrated safety and effectiveness as a CAMP therapy, promoting rapid wound size reduction. Future studies evaluating larger patient populations will be necessary.