Introduction
Diabetic foot ulcers (DFUs) represent one of the most serious and disabling complications of diabetes mellitus, resulting from the combined effects of excessive plantar pressure, poorly fitting footwear, gait abnormalities, and repetitive mechanical trauma.1 The clinical evaluation and classification of DFUs typically incorporate ulcer dimen sions and depth, infection status, vascular and neuropathic involvement, and anatomical distribution.2
Globally, DFUs affect approximately 19-34% of individuals with diabetes and are associated with a five-year mortality rate between 50% and 70%.1 Despite established management protocols, outcomes remain suboptimal. The standard of care (SOC), which includes regular sharp debridement, offloading, bacterial burden reduction, and maintenance of a moist wound environment, achieves complete closure in fewer than half of patients within 12 weeks.3
Preventive measures, such as periodic screening and multidisciplinary coordination between primary and specialty care, are central to reducing DFU incidence but are often resource-intensive and costly.4 An analysis looking at the economic burden associated with DFUs using 2007-2011 United States data, estimated costs to be $11,000-$17,000 higher per patient compared with individuals with diabetes but without DFUs.5
Advanced wound management approaches increasingly employ cellular, acellular, and matrix-like products (CAMPs), defined as “a broad category of biomaterials, synthetic materials, or biosynthetic matrices that support the repair or regeneration of injured tissues through various mechanisms of action”.6 CAMPs provide several therapeutic benefits, including protection of the wound microenvironment, coverage of exposed structures, facilitation of surgical closure, and enhancement of both functional and cosmetic outcomes.6
Materials and methods
CAMPX is a randomized controlled multicenter clinical trial designed to evaluate the efficacy of a single layer amniotic membrane (SLAM; XWRAP®, Applied Biologics™, Scottsdale, AZ, USA) with standard of care (SOC) compared with SOC alone for the treatment of nonhealing diabetic foot ulcers (DFUs) (clinicaltrials.gov #NCT06564831). This study was conducted at 19 SerenaGroup®, Inc. or affiliated centers throughout the United States with 216 patients with nonhealing DFUs. At the time of interim analysis, enrollment began April 2024 and interim analysis was conducted October 2025. The study population was drawn from patients suffering from chronic wounds who were attending wound clinics.
Objectives and endpoints
The primary objective for the CAMPX clinical trial was to determine the efficacy of SLAM plus SOC versus SOC alone in achieving complete closure of nonhealing diabetic foot ulcers over 12 weeks. The primary endpoint was the percentage of target ulcers achieving complete wound closure in 12 weeks.
An additional important endpoint evaluated was percentage wound area reduction from TV-1 to TV-13 measured weekly with digital photographic planimetry and physical examination.
Diagnosis
The diagnosis of DFUs is predominantly clinical, based on a detailed patient history, physical examination, and targeted diagnostic testing. These ulcers most commonly develop in weight-bearing regions of the foot and are frequently preceded by signs of peripheral neuropathy and peripheral arterial disease (PAD).
Neuropathic ulcers are typically characterized by a demarcated, punched-out appearance with a callused border and variable granulation tissue. In contrast, ischemic and neuroischemic ulcers often display irregular wound margins, pallor or necrosis of the wound bed, and minimal exudate. Pain sensation is usually diminished or absent due to sensory neuropathy but may be more pronounced when ischemia or secondary infection is present.
A thorough medical history is crucial for distinguishing DFUs from other chronic ulcerations. Important historical details include the duration and control of diabetes, history of previous ulceration or amputation, presence of peripheral vascular disease, neuropathic symptoms, mechanical or traumatic precipitating factors, prior wound management strategies, and footwear practices. Differential diagnoses to consider and exclude include venous leg ulcers, non-diabetic arterial ulcers, pressure injuries, vasculitic lesions, and malignant ulcerations.
Neurological evaluation was performed to assess for loss of sensation. Vascular assessment was conducted in all prospective subjects, primarily using the ankle-brachial index (ABI). Individuals with an ABI greater than 0.7 were eligible for inclusion, whereas values exceeding 1.3 prompted further vascular assessment. In patients with noncompressible, calcified arteries, alternative methods such as the toe-brachial index (TBI) were utilized, with TBI values ≥0.6 indicating satisfactory perfusion. Additionally, a transcutaneous oxygen measurement (TCOM) measurement of ≥40 mmHg was considered evidence of adequate tissue perfusion for inclusion.
Vulnerable populations
Although vulnerable subjects were not specifically recruited for this study, vulnerable subjects were present in the potential subject pool.
Product description
This study evaluated a single layer amniotic membrane (SLAM; XWRAP®, Applied Biologics™, Scottsdale, AZ, USA), a human amniotic membrane allograft designed as a protective barrier for DFUs, venous leg ulcers (VLUs), and pressure ulcers. Processing methods preserve the structural integrity of the epithelial membrane, and all donor material undergoes stringent screening and testing in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory meeting or exceeding American Association of Tissue Banks (AATB) standards. SLAM is regulated under FDA 21 CFR 1271 and AATB Standards, with processing performed in an FDA-registered, AATB-accredited facility.
Each graft is sterile-packaged for single-patient, single-use application, and includes a Tissue Utilization Record with chart labels.
Subject characteristics
Patients who suffer from chronic DFUs were recruited for this study from participating wound clinics. Once patients agreed to adhere to the study schedule, and read and sign the IRB approved Informed Consent Form, screening was conducted to determine whether subjects were eligible based on inclusion and exclusion criteria, listed in Table 1.
TABLE 1 Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Study procedures
Subjects followed a structured series of clinical visits which included screening, treatment, closure verification, and follow-up phases. This schedule was designed to ensure assessment of eligibility, uniform application of wound care procedures, consistent administration of study interventions, and accurate evaluation of study endpoints (Table 2).
TABLE 2 Study schedule
| SV | TV-1 | TV-2 | TV-3 | TV-4 | TV-5 | TV-6 | TV-7 | TV-8 | TV-9 | TV-10 | TV-11 | TV-12 | TV-13 | CCV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Window Period | -14 | Day 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | +14 |
| Record medical history and demographic information | X | X | |||||||||||||
| Sign consent | X | ||||||||||||||
| Randomization | X | ||||||||||||||
| HbA1c | X | ||||||||||||||
| Wound assessment | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Physical exam | X | X | |||||||||||||
| Vascular screening test | X | ||||||||||||||
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Assessment for AE and SAE | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Review medication changes | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Treatment based on randomization | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Study ulcer area with digital images | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Study ulcer cleaning, debridement (if applicable) | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Pain assessment (PEG) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Protease swab | X | X | X | ||||||||||||
| Wagner grade and Fitzpatrick scale | X | ||||||||||||||
| Historical measurement | X | ||||||||||||||
| Fluorescence imaging | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Apply dressing | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Offloading (if a plantar wound) | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Quality of Life wQOL and FWS | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Mini Nutrition Assessment (MNA) | X | ||||||||||||||
| CGM | X | X | X | X | X | X | X | X | X | X | X | X | X |
During the screening visit, informed consent was obtained, and medical history was reviewed to confirm eligibility. Demographic data were collected, along with medical and medication histories. Vascular screening was performed unless results from the previous three months were available. Vital signs were recorded, and a general physical examination was conducted. Additional assessments included the Mini Nutritional Assessment (MNA), HbA1c documentation (using results from the past three months if available), Wagner Grade, Fitzpatrick Skin Type Scale, and Pain Assessment using the PEG tool.
Wound evaluation included documentation of granulation tissue, nonviable tissue, wound depth, exudate, and condition of the periwound skin. Historical wound measurements from the two weeks preceding the screening visit were also recorded. A reduction in wound size exceeding 20% during this historical run-in period was considered a screen failure. Each ulcer was photographed, and surface area was measured using an imaging device following debridement. Baseline fluorescence imaging was performed to detect bacterial fluorescence in the wound bed or periwound area.
During the 14-day screening phase, SOC procedures were implemented, including wound cleansing with normal sterile saline (NSS) and sharp debridement. Calcium al-ginate or foam dressings were applied, and no other dressings were permitted without prior approval from the medical monitor. Offloading was initiated for plantar ulcers or wounds exposed to external pressure, using a removable walker boot as the preferred method or total contact casting (TCC) when necessary. Continuous glucose monitoring (CGM) was initiated, and patients were instructed in its use.
At the enrollment visit, approximately 14 days after screening, eligibility criteria were re-confirmed. Adverse events were assessed, medications reviewed, and a symptom-directed physical examination was completed. Vital signs were recorded, and wound characteristics reassessed. The percentage area reduction (PAR) over the screening period was reviewed and had to remain below 20% for continued eligibility. The ulcer was photographed and measured with an imaging device after debridement. Pain and quality-of-life were assessed using the PEG, Forgotten Wound Score (FWS), and wound-specific Quality of Life (wQOL) instruments. Fluorescence imaging was per-formed to document bacterial presence, and CGM data were downloaded to calculate time-in-range (TIR) values. Subjects who continued to meet eligibility criteria were randomized electronically and treated according to their assigned study arm. SOC included wound cleansing with NSS, debridement as indicated, and application of calcium alginate or foam dressing provided by the study. Dressing deviations were not permitted. Offloading adherence was evaluated in subjects not using TCCs.
Subjects attended weekly visits until week 12 or wound closure, whichever occurred first. At each visit, adverse events were assessed, medications reviewed, and vital signs recorded. Wound characteristics, including tissue type, depth, exudate, and periwound condition, were documented. Wounds were measured using an imaging device following debridement, and pain and quality-of-life were assessed via PEG, wQOL, and FWS instruments. Fluorescence imaging was repeated, and protease swabs were collected at treatment visit (TV)-5 and TV-9. CGM data were downloaded, and TIR values recorded. SOC treatment included cleansing with NSS, sharp or mechanical debridement at the investigator's discretion, and fluorescence imaging post-debridement. The ulcer was treated according to the assigned arm (SLAM + SOC or SOC only) and dressed with calcium alginate or foam dressing. For wounds with excessive exudate, additional absorptive layers were permitted with medical monitor approval. Offloading adherence was again assessed for plantar ulcers.
At the final treatment visit (week 13) or earlier if closure occurred prior to week 12, medications and adverse events were reviewed, and pain and quality-of-life assessments were repeated. For open wounds, detailed wound characteristics were recorded, and fluorescence imaging and wound measurements were performed. Protease swabs were collected, and non-healed subjects were referred for continued wound care. End-of-study documentation was completed for all participants.
Two weeks (±3 days) after initial assessment of complete re-epithelialization, subjects returned for a Closure Confirmation Visit. This visit occurred even if closure was first observed at the final study visit. At the CCV, investigators reviewed adverse events and concomitant medications, assessed pain status, and confirmed closure using an imaging device. Complete wound closure was verified and documented.
Subjects who withdrew or were withdrawn by the investigator prior to study completion underwent all procedures described for the final treatment visit. Unscheduled visits, such as those for additional dressing changes, were documented by the study coordinator in the CRF, including the reason for the visit. During such visits, adverse events and concomitant medications were reviewed, and secondary dressings were replaced as necessary.
At study exit, subjects with unhealed wounds returned to their physician for continued SOC management. No further follow-up procedures or therapy transitions were required beyond the study period. In addition to the principal investigator's assessment of wound closure, an independent evaluation of the primary endpoint was performed. Two independent reviewers, blinded to study arm assignment, reviewed deidentified digital images obtained at the closure and confirmation visits using the imaging device. Reviewers classified each image as “healed” or “not healed”. In cases of disagreement, the assessment that aligned with the principal investigator’s determination was used for final analysis.
Subject withdrawal
All participants had the right to withdraw from the study at any time during the treatment period without prejudice. The completion status of each participant’s involvement in the clinical trial was documented. In the event that study treatment or protocol-required observations were discontinued for any participant, the reason(s) for discontinuation were recorded. The investigator had the authority to withdraw a participant from the study at any time if deemed medically necessary. Whenever feasible, the reason for withdrawal or early termination was documented.
A participant was classified as lost to follow-up if they could not be reached after five telephone contact attempts and three written communications.
Subject compensation
Participants received a nominal compensation of USD 50 upon completion of each study visit. This compensation was intended to offset expenses associated with participation, including travel, parking, and the additional time required for study-specific procedures and data collection.
Results
A total of 216 DFU patients from multiple sites were included in the interim analysis. 57 patients received standard of care alone, and 61 patients received SLAM plus standard of care. 15 patients from both treatment groups are considered ongoing at the time of interim analysis. 11 patients were discontinued during the study and 74 were excluded during screening. Summary statistics on demographic variables are provided in Table 3.
TABLE 3 Demographic summary statistics by treatment group
| Variable | Standard of Care | SLAM | P-Value |
|---|---|---|---|
| Age (Years) | |||
| N (Mean) | 57 (62.7) | 61 (62.0) | 0.726 |
| Gender, N (%) | |||
| Male | 45 (78.9) | 45 (73.8) | 0.657 |
| Female | 12 (21.1) | 16 (26.2) | |
| Fitzpatrick Scale, N (%) | |||
| Type I | 10 (17.5) | 12 (19.7) | 0.024 |
| Type II | 26 (45.6) | 13 (21.3) | |
| Type III | 14 (24.6) | 19 (31.1) | |
| Type IV | 4 (7.0) | 7 (11.5) | |
| Type V | 3 (5.3) | 3 (4.9) | |
| Type VI | 0 (0) | 7 (11.5) | |
| Wagner Grade, N (%) | |||
| Grade 0 – no ulcer | 0 (0) | 0 (0) | 0.554 |
| Grade 1 – superficial ulcer | 43 (75.4) | 42 (68.9) | |
| Grade 2 – deep ulcer | 14 (24.6) | 19 (31.1) | |
| Grade 3 – ulcer + cellulitis/ osteomyelitis | 0 (0) | 0 (0) | |
| Grade 4 – localized gangrene | 0 (0) | 0 (0) | |
| Grade 5 – extensive gangrene | 0 (0) | 0 (0) | |
| Race, N (%) | |||
| American Indian/Alaskan Native | 0 (0) | 0 (0) | 0.002 |
| Asian | 1 (1.8) | 0 (0) | |
| Black | 4 (7.0) | 20 (32.8) | |
| Pacific Islander | 0 (0) | 0 (0) | |
| White | 50 (87.7) | 41 (67.2) | |
| Other | 2 (3.5) | 0 (0) | |
| Current Tobacco Use, N (%) | |||
| Yes | 3 (5.3) | 7 (11.5) | 0.379 |
| No | 54 (94.7) | 54 (88.5) | |
No statistically significant differences were observed across treatment groups (all p > 0.05) except race and Fitzpatrick Scale, suggesting that randomization achieved adequate baseline balance. Wound area, wound age, and patient age were used as stratification factors in the trial design, and at this interim analysis, they are summarized descriptively to assess balance, shown in Table 4. The reported p-values are exploratory checks of randomization balance and were not used to adjust the interim analysis endpoints.
TABLE 4 Stratification summary statistics
| Variable | Standard of Care (n = 57) | SLAM (n = 61) | P-value |
|---|---|---|---|
| Wound area, N (%) | |||
| Less than 2cm2 | 23 (40.4) | 21 (34.4) | 0.336 |
| Greater than 2cm2 | 34 (59.6) | 38 (62.3) | |
| Wound age, N (%) | |||
| Wound >6 months | 33 (57.9) | 31 (50.8) | 0.327 |
| Wound <6 months | 24 (42.1) | 28 (45.9) | |
| Patient Age, N (%) | |||
| Patient > 65 Years Old | 25 (43.9) | 25 (41.0) | 0.382 |
| Patient < 65 Years Old | 32 (56.1) | 34 (55.7) | |
The primary endpoint was assessed for the interim analysis. The primary endpoint is the percentage of target ulcers achieving complete wound closure in 12 weeks. Additionally, the percent area reduction (PAR) from TV-1 to TV-13 measured weekly with digital photographic planimetry, using an imaging device, and physical examination were analyzed. The intent-to-treat (ITT) and per protocol (PP) populations were analyzed.
In the ITT population, the SLAM + SOC arm achieved a 13.1% closure rate versus 8.8% with SOC alone. Among the PP population, closure rose to 20% on SLAM + SOC versus 16% on SOC.
Within each arm, any individual PAR value falling below Q1 – 1.5*IQR or above Q3 + 1.5*IQR was flagged and excluded. For ITT, SLAM + SOC outperformed SOC on both average and median wound-area reduction, with a mean PAR ~33% versus ~30% (without outliers). Summary statistics are provided in Table 5.
TABLE 5 Percent area reduction (PAR) summary statistics without outliers for ITT.
| Treatment arm | n | Mean | Standard deviation | Median | IQR |
|---|---|---|---|---|---|
| Standard of Care | 56 | 29.96 | 52.77 | 26.1 | 80.14 |
| SLAM + SOC | 56 | 32.94 | 47.69 | 38.59 | 64.89 |
For PP, SLAM + SOC outperformed Standard of Care on both average and median wound-area reduction, with summary statistics for each treatment group (without outliers) reported in Table 6.
TABLE 6 Percent area reduction (PAR) summary statistics without outliers for PP.
| Treatment arm | n | Mean | Standard deviation | Median | IQR |
|---|---|---|---|---|---|
| Standard of Care | 25 | 41.03 | 60.84 | 59.5 | 107.91 |
| SLAM + SOC | 24 | 56.3 | 49.11 | 69.24 | 71.7 |
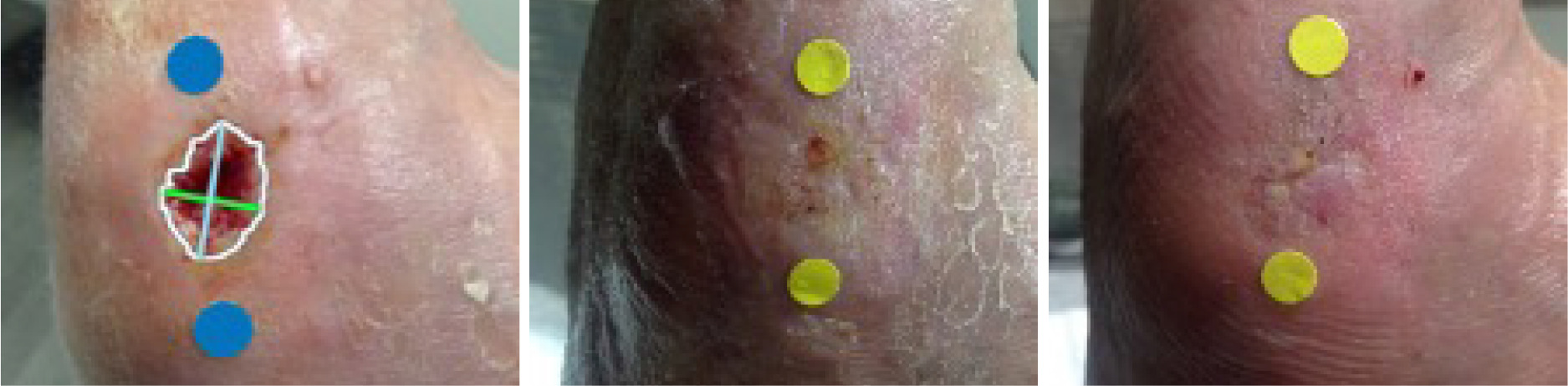
Sequential images shown in Figure 1 document the trajectory of wound healing from TV-1, TV-11, and HCV in a patient assigned to the SLAM with SOC treatment arm
FIGURE 1 Digital images from TV-1, TV-11, and HCV (left to right), SLAM with SOC treatment arm. Patients gave consent for publication of images.
Discussion
Interim analysis included a data lock on the electronic data capture (EDC) system and quality assurance review prior to data analysis. The purpose of this interim analysis is to determine balance across treatment groups and comparison to current standard of care for the primary endpoint and PAR. Patients were stratified by wound area, wound age, and patient age. There is no significant difference between strata between treatment groups, therefore, the randomization scheme achieved a balanced baseline. Additional analysis by the stratification group is planned for the final analysis.
Conclusion
For the primary endpoint, SLAM + SOC achieved numerically higher closure rates in both ITT and PP populations. In ITT, the relative risk was 1.49 (95% CI 0.52-4.30), while in PP, the relative risk was 1.25 (95% CI 0.38 – 4.12). The small differences in sample size between populations may influence the results of the Chi-squared test, and additional enrollment will occur until the planned sample size is met for all treatment groups.
Percent area reduction provides insight into the closure rates by treatment group. In the ITT and PP population, SLAM + SOC achieved a higher mean area reduction than SOC. This provides promising results at interim and confirmation of the clinical trial design prior to final analysis.