Introduction
Peripheral arterial disease (PAD) is a growing public health concern, impacting more than 8.5 million people in the US, and is closely associated with diabetes, chronic kidney disease, and cardiovascular morbidity and mortality.1,2 Early and accurate identification of PAD is crucial to prevent severe clinical outcomes such as chronic limb-threatening ischemia (CLTI), ulcers, infections, and potential amputations.3 Ankle-brachial index (ABI) is the most widely used screening tool for PAD, measuring the ratio between ankle and brachial systolic pressure. Despite its widespread adoption, the ABI often yields unreliable results in specific patient populations, particularly those with diabetes mellitus or advanced age. This is due to arterial calcification and subsequent vessel incompressibility that lead to falsely elevated or non-diagnostic readings.3,4 Moreover, ABI testing requires training, technical competence, and patient cooperation. Its use in primary care is often limited by the need for Doppler equipment and cuff positioning expertise.4
Additionally, the ABI is not feasible in the clinical setting of open wounds or bypass grafts, in which non-invasive assessment is critical. This also holds true for pulse volume recordings (PVR).
Alternative assessments, such as the toe-brachial index (TBI) and transcutaneous oxygen pressure (TcPO2), present their own limitations in cost, reproducibility, and accessibility.5 Consequently, there remains a significant unmet need for a quick, reliable, simple, and accurate diagnostic alternative. Near-infrared spectroscopy (NIRS) has emerged as a promising, non-contact method to evaluate superficial tissue perfusion by measuring the relative absorption of oxygenated and deoxygenated hemoglobin in real time.6 Devices like SnapshotNIR provide portable high-resolution imaging of tissue oxygen saturation (StO2) across wound beds and extremities.7
The plantar-palmar index (PPI) introduced in this study is calculated as the ratio of StO2 in the plantar foot compared to the palmar hand. The authors hypothesize the PPI may reflect lower extremity perfusion relative to a stable upper extremity reference, analogous to the ABI but with increased adaptability and without the need for cuff-related blood pressure measurement and Doppler skills.
NIRS has emerged as an innovative diagnostic modality capable of assessing real-time tissue oxygenation by detecting and differentiating oxygenated and deoxygenated hemoglobin concentrations within superficial tissues.6 Advances in NIRS imaging technology, specifically non-contact devices like SnapshotNIR, allow expedient point-of-care evaluations without patient discomfort or complex procedural requirements.7 Previous research has validated the application of NIRS in clinical decision-making including in surgical procedures, wound care, flap viability assessments, critical limb ischemia management and diabetic wound healing prediction.8-10
To address the limitations inherent in the ABI, this study introduces and evaluates a novel diagnostic parameter, the plantar-palmar index (PPI). The PPI leverages the NIRS-derived tissue oxygen saturation values (StO2) from plantar and palmar surfaces, offering clinicians an objective measure of lower-extremity perfusion against a consistent upper extremity baseline.
The objective of this study was to evaluate whether the PPI can serve as an accurate, reproducible surrogate for ABI and correlate with standard diagnostic tools such as PVR. The study aims to determine if PPI correlates with PAD severity, defined by PVR, and to validate its potential as a practical, accurate, and clinically feasible alternative diagnostic tool.
Methods
Study population
Data were collected from a total of 91 limbs from 48 subjects in the setting of a wound care center population including patients with diabetes, known PAD, healed or active ulcers, and healthy controls.
Ethical approval and patient consent
Informed consent was obtained from all participants. The study protocol received IRB approval (Advarra Pro00080566) and was conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as amended in 2013.
Protocol
ABI and PVR
All limbs were assessed using the MESI mTABLET ABI system.
NIRS imaging
Tissue oxygen saturation was measured using the SnapshotNIR device (model KD205, Kent Imaging, Calgary). Images of the plantar and palmar surfaces were obtained at supine position. The environment was standardized by using the same room, lighting and temperature control. Images were centered and focused on the metacarpal and metatarsal region of interest (ROI) and an average of the tissue StO2 values were obtained and recorded from these ROIs using the circumscribing feature of the device (Figure 1). A modified Buerger’s test (provocative elevation maneuver of the lower extremity – PEMLE) was then performed. Each leg was raised to 45 degrees while the patient was supine. A second NIRS image was captured at 60 seconds of elevation of the metatarsal ROI and the StO2 value was recorded.
FIGURE 1 Region of Interest for Plantar-palmar index (PPI).
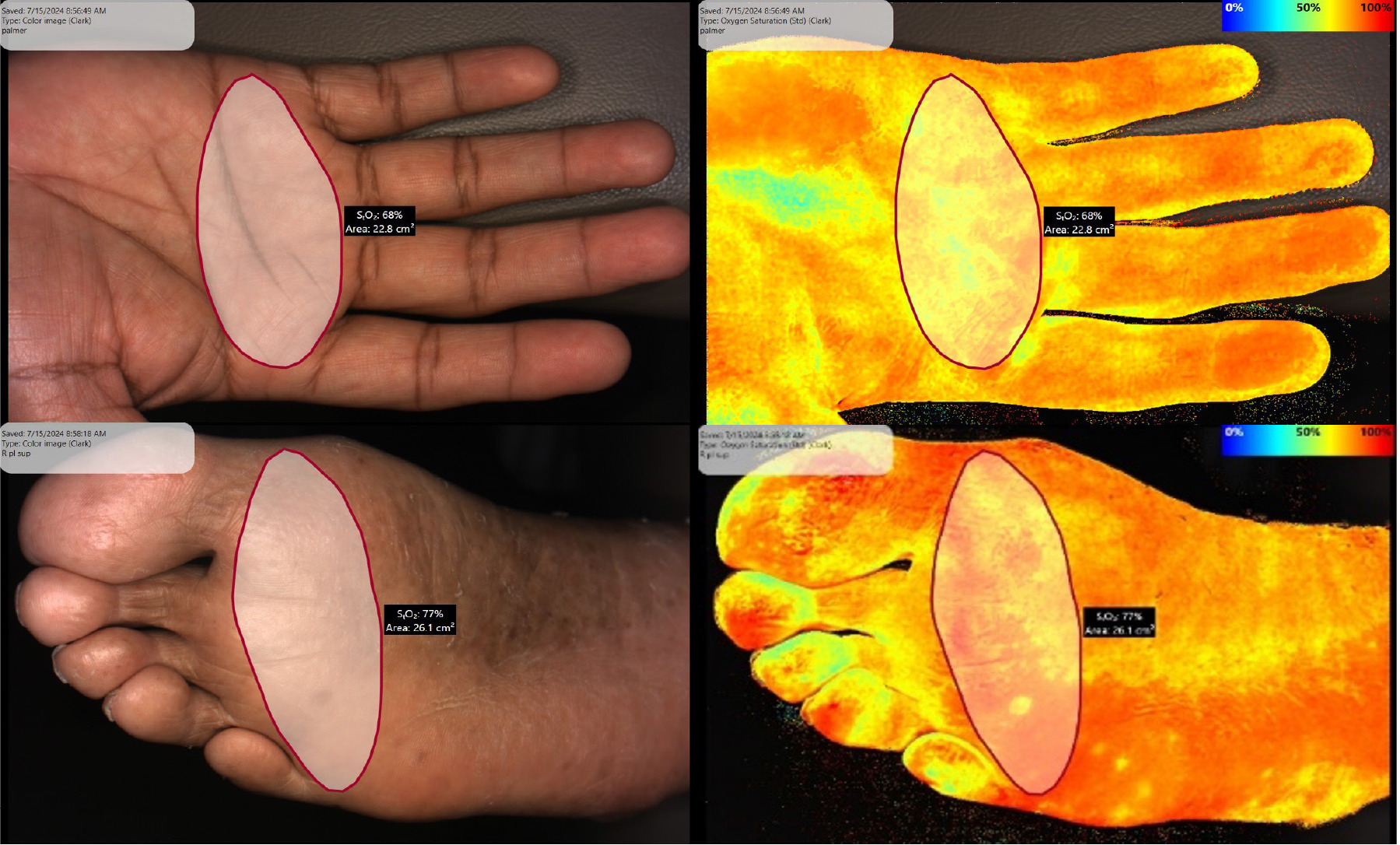
Plantar-palmar index calculation
The PPI was calculated based on the recorded StO2 values using the following formula:
PPI = Plantar StO2 / Palmar StO2
Statistical analysis
Limbs were grouped into normal, mild, moderate, and severe PAD categories based on PVR waveforms. Statistical analyses were conducted using SPSS (version 29.0.2.0). Data are reported as mean ± standard deviation. Between-group comparisons are expressed as estimated means (adjusted group means taking into account covariates) with 95% confidence intervals (CIs) providing a range within which the true population mean is likely to fall. A p-value of less than 0.05 was considered statistically significant. Data normality was evaluated using the Shapiro-Wilk test. To compare NIRS measurements across varying levels of PAD severity (normal, mild, moderate, and severe), a one-way independent measures analysis of variance (ANOVA) was employed. When significant differences were found, post-hoc pairwise comparisons were performed using least significant difference (LSD) test.
Results
Demographic data are summarized in Table 1. A total of 91 limbs from 48 patients were included in the final analysis. Among these, based on PVR, 49 limbs were classified as normal, 32 with mild disease, 7 with moderate disease, and 3 with severe disease. Study subjects represented Hispanic, Black and Caucasian ethnicities, with Fitzpatrick skin types II-VI.
TABLE 1 Demographic information
| Peripheral arterial disease (PAD) severity (no. of limbs) | ||||
|---|---|---|---|---|
| Variable | Normal (49) | Mild (32) | Moderate (7) | Severe (3) |
| Age (years) (mean ± SD) | 63 ± 15 | 70 ± 9 | 77 ± 11 | 75 ± 3 |
| Comorbidities: | ||||
| History of PAD | 4 (8%) | 7 (22%) | 3 (43%) | 3 (100%) |
| History of vascular intervention | 0 (0%) | 2 (6%) | 1 (14%) | 0 (0%) |
| History of ulcer | 10 (20%) | 12 (4%) | 4 (57%) | 2 (67%) |
| History of diabetes | 19 (39%) | 11 (3%) | 5 (71%) | 1 (33%) |
| History of smoking | 23 (47%) | 22 (69%) | 5 (71%) | 3 (100%) |
While supine resting plantar StO2 showed no group difference for different PAD groups (Figure 2 - left), the PPI values increased with disease severity (Figure 2 - right).
FIGURE 2 Plantar StO2 across peripheral arterial disease (PAD) groups (left) and plantar-palmar index (PPI) across PAD groups (right).
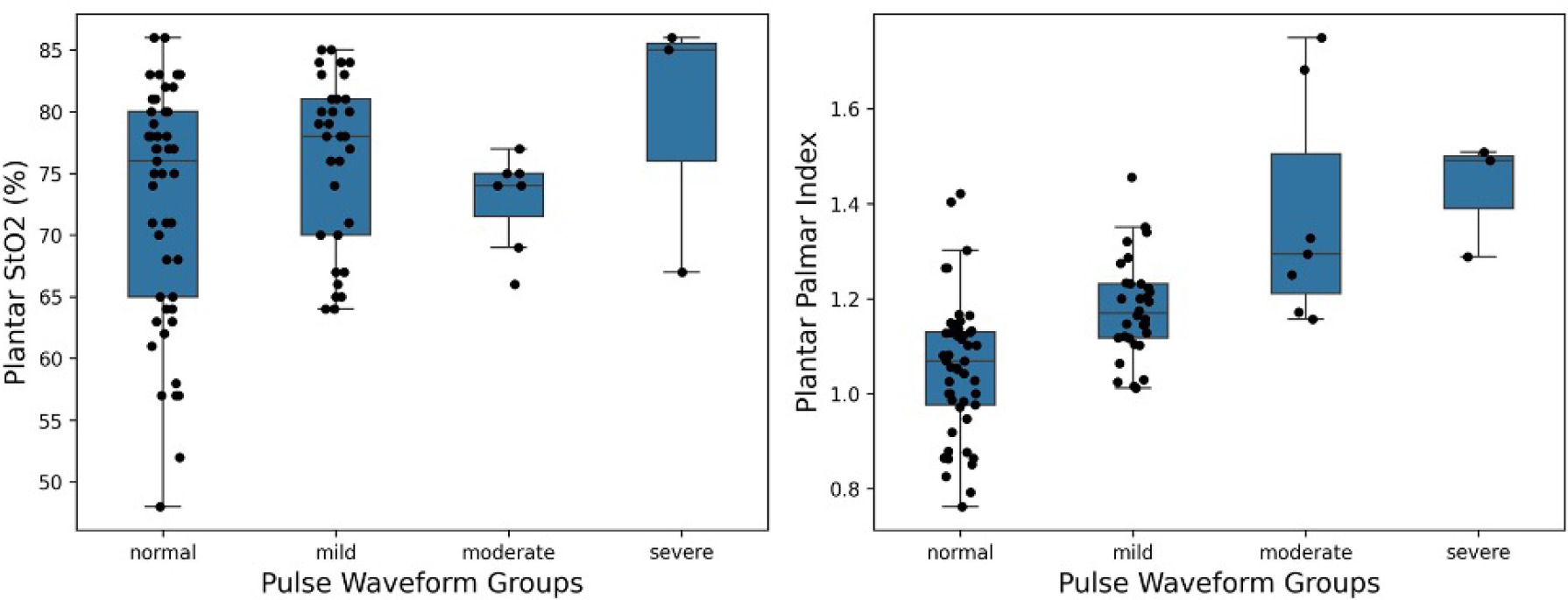
ANOVA confirmed significant differences among groups (<0.001) with estimated means for normal (1.054; 95% CI, 1.015–1.094), mild (1.178; 95% CI, 1.129–1.227), moderate (1.376; 95% CI, 1.271–1.481), and severe (1.430; 95% CI, 1.270– 1.59) (Table 2). Pairwise comparisons showed significant differentiation between normal and mild (p<0.001), normal and moderate (p<0.001), normal and severe (p<0.001), mild and moderate (p<0.001) and mild and severe (p<0.005), except for moderate and severe (p=0.574) PAD groups (Figure 3). ΔStO2 for plantar surface between supine resting and elevated position showed significant group difference in ANOVA as well (p=0.003). Post-hoc Tukey test revealed that only the moderate group was significantly different from controls (p<0.001) and the mild group (p=0.007).
FIGURE 3 Estimated mean and 95% CI for plantar-palmar index across peripheral arterial disease groups.
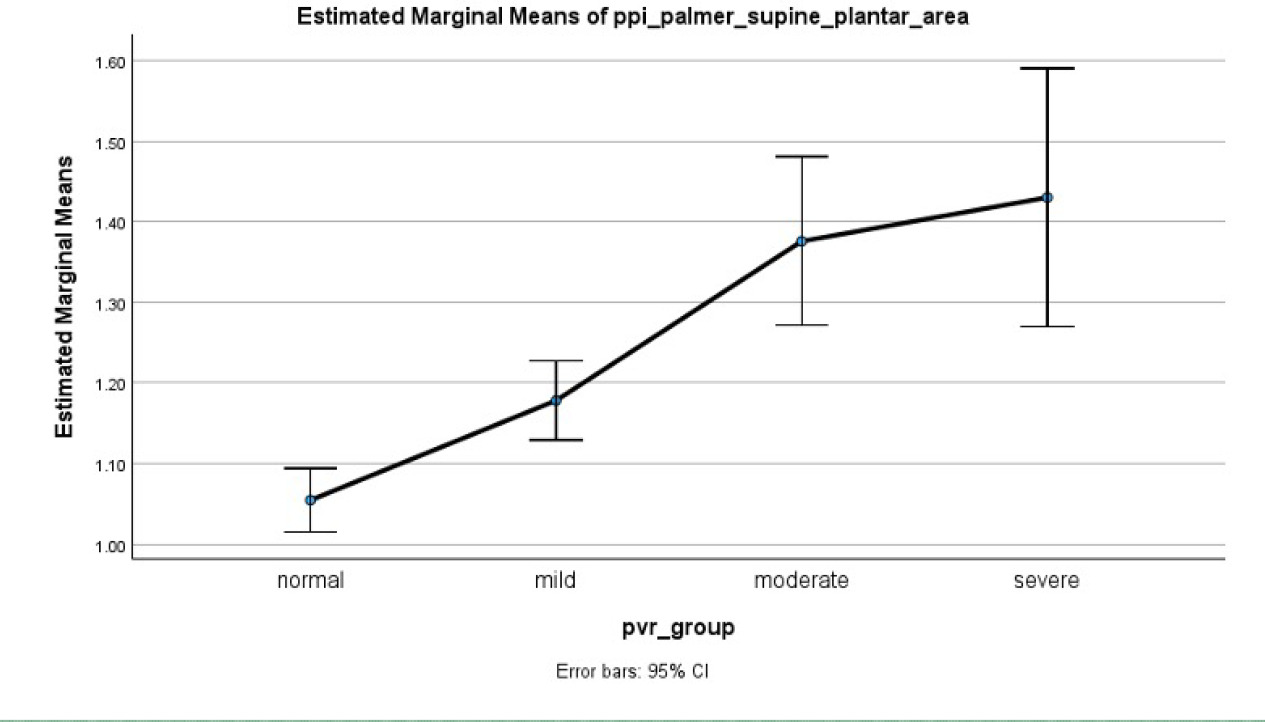
TABLE 2 Estimated mean plantar-palmar index (PPI) by pulse volume recording (PVR) classification
| PVR classification | Estimated mean PPI (95% CI) |
|---|---|
| Normal | 1.054 (1.015–1.094) |
| Mild peripheral arterial disease (PAD) | 1.178 (1.129–1.227) |
| Moderate PAD | 1.376 (1.271–1.481) |
| Severe PAD | 1.430 (1.270–1.590) |
Discussion
The ABI and PVR tests are both used to assess blood flow in the legs and evaluate PAD. ABI compares blood pressure in the ankle to the arm, while PVR measures blood flow by recording changes in blood volume. The clinical limitations of the ABI are particularly evident in diabetic populations, where calcified vessels result in non-compressible arteries and falsely elevated indices.3 Studies show ABI fails to detect early disease and offers little insight into dermal microcirculation.8 PVR is more sensitive than ABI and provides more detailed information about the nature and severity of arterial stenosis, especially in cases of calcified arteries. In contrast, NIRS evaluates superficial tissue perfusion and is unaffected by calcification.9
This study demonstrates that PPI, derived from non-contact NIRS, aligns closely with PVR-defined PAD severity. PVR, similar to ABI, requires tissue contact with attendant limitations. Prior studies support the value of NIRS technology in wound assessment, flap monitoring, and critical limb ischemia.10,12. For instance, Gopalakrishnan et al showed how NIRS accurately tracked tissue perfusion recovery in CLI patients post-revascularization.11 Unlike ABI and PVR, NIRS can be performed expeditiously at the point of care, with no physical contact or need for specialized equipment or training. SnapshotNIR has also demonstrated usability in intraoperative settings, flap viability assessment, and surgical wound planning.14-15
PPI’s ability to consistently distinguish between severity classes suggests its strong potential as a front-line PAD screening tool – especially in high-risk or resource-limited environments. It may also play a critical role in post-revascularization monitoring and guiding therapy escalation.
The findings from this study clearly demonstrate that the PPI, calculated using NIRS, strongly associates with established PAD severity classifications derived from PVR. Notably, mean PPI values significantly increased in direct proportion to PAD severity, effectively distinguishing between normal and PAD-affected groups. These findings underscore the potential utility of PPI as an accurate, objective, and clinically feasible alternative to ABI.
The clinical challenges posed by ABI are extensively documented, particularly its limitations in diabetic and elderly populations due to calcified vessels, resulting in non-compressible arteries and consequently unreliable or misleading readings.3,4 The widespread presence of diabetes and chronic renal insufficiency among PAD patients further emphasizes the clinical imperative for reliable diagnostic alternatives, as early and accurate PAD detection is essential for optimal patient management and outcomes.2,3,8
NIRS imaging directly evaluates dermal tissue perfusion at a microvascular level, bypassing the limitations associated with arterial calcification and offering an advantage over traditional pressure-based diagnostic tests like ABI or toebrachial index (TBI).6,8 In clinical settings, this modality has demonstrated considerable value in wound assessment.8,10 For example, Andersen et al demonstrated the superiority of NIRS imaging over visual inspection for objectively defining complete wound healing and predicting wound recurrence, highlighting the practicality of NIRS in guiding clinical decision-making in real-world settings.9 Additionally, Gopalakrishnan et al confirmed that NIRS effectively monitors patients with critical limb ischemia and ischemic ulcers, providing real-time tissue oxygenation data crucial for clinical decision-making and therapeutic monitoring.10
The PPI introduced in this study significantly enhances the clinical applicability and interpretation of NIRS data, simplifying assessment and providing clinicians with an intuitive index analogous to ABI, but without the methodological constraints of arterial compression or attempting to place cuffs over wounded tissue.7,10 This improved accessibility and ease of use make the PPI particularly attractive for clinical practice, especially in wound care and outpatient vascular clinics where efficient and reliable diagnostic information is invaluable. A progressive decline in palmar StO2 was observed with increasing PAD severity, which contributed to an elevation in PPI in the higher severity cohorts. The ΔStO2 from the leg elevation maneuver also demonstrated its usefulness to differentiate the moderate diseased group from control and mild groups. Increasing the number of samples in the severe group could be helpful to obtain a clearer distinction for that group.9
Future research should aim to validate the identified range of PPI values in larger, multicenter trials and diverse clinical populations to confirm its diagnostic reliability and further delineate clinical recommendations and guidelines. Combining the limb elevation maneuver with PPI might help to enhance diagnostic ability as well. Establishing clear clinical thresholds and integrating NIRS-based PPI into current vascular diagnostic protocols could significantly enhance PAD detection, patient stratification, and therapeutic decision-making, ultimately improving patient outcomes and reducing PAD-associated morbidity.
Other publications identify that a significant benefit of NIRS is its ability to provide bedside assessment of tissue oxygenation.16 In non-healing wounds this allows more frequent assessment of the patient responses to treatment protocols. These reassessments in turn allow the provider to pivot quickly with tailored adjustments to the plan of care. The PPI represents a distinct functionality of NIRS as an alternative to other non-invasive vascular tests but should not be viewed to diminish other values of the technology, which includes trending response to conservative and advanced therapies over weeks of treatment.
Conclusion
The PPI, derived from NIRS imaging, correlates significantly with PAD severity as defined by PVR. It provides an efficient, non-invasive alternative to ABI, particularly in populations where ABI is limited by vascular calcification. NIRS-based assessment may reshape clinical workflows in vascular diagnostics and wound care, offering improved patient stratification and treatment monitoring. Further studies with larger, diverse populations are warranted to validate these findings and establish standardized thresholds for PPI interpretation and integration into management protocols in vascular and wound care settings.