Foreword
The rapid evolution of imaging technologies has transformed our approach to managing surgical wounds. Among these innovations, fluorescence imaging stands out as a tool with profound implications for clinical practice, patient outcomes, and healthcare systems at large. The integration of point-of-care fluorescence imaging can reshape clinical workflows by providing objective, real-time insight into bacterial burden.
This manuscript represents a consensus-driven, multidisciplinary effort to capture the clinical utility, procedural integration, and emerging standards surrounding the use of fluorescence imaging throughout the surgical care continuum. From pre-operative assessment to intra-operative precision and post-operative surveillance, the chapters that follow illustrate how this technology is helping to close the diagnostic gap that has historically hindered timely and appropriate interventions for bacterial burden.
In an era marked by increasing antimicrobial resistance, patient complexity, and medicolegal scrutiny, the ability to visualize pathogenic threats before they manifest as clinical complications is no longer a luxury but a necessity. It is my hope that this manuscript will serve as both a guide and a catalyst for further adoption, investigation, and refinement of fluorescence imaging in surgical practice. The evidence and opinions expressed here are compelling. Fluorescence imaging has the potential to improve outcomes in surgical patients across the globe.
Dr. Thomas E. Serena. Chairman, Consensus Panel
Introduction
Undetected high bacterial loads are a significant barrier to wound healing, delaying recovery, complicating treatment strategies, and contributing to increased healthcare costs.1 These elevated bacterial levels, often undetectable through visual inspection or traditional clinical signs, can persist in surgical and chronic wounds without outward symptoms, silently impeding healing progress and increasing the risk of surgical site infections (SSIs), graft failure, and wound dehiscence.1
Current diagnostic approaches, including clinical signs and symptoms (CSS), wound cultures, and swab analyses, fail to identify the full extent of bacterial colonization, particularly in the early or subclinical stages. In a multicenter analysis, 93.9% of wounds had bacterial loads exceeding 104 CFU/g, yet clinical symptoms of infection were largely absent.2 This diagnostic gap poses a significant threat to timely and effective intervention, especially in high-risk patients with comorbidities such as diabetes, peripheral vascular disease, or compromised immune function.
In this context, fluorescence imaging has emerged as a promising point-of-care solution. It is rapidly becoming a standard of care in outpatient wound centers due to its ability to enhance early bacterial detection and improve clinical outcomes. The MolecuLight® Imaging Device (Toronto, Canada) is currently the most widely adopted tool for this purpose. It provides real-time, non-contact visualization of bacterial presence by emitting a safe violet (405 nm) light. This light excites endogenous fluorophores (porphyrins and pyoverdines) produced by metabolically active bacteria. When excited, these fluorophores emit visible fluorescence in red and cyan wavelengths, which correspond to different types of bacteria when present at loads above 104 CFU/g: red fluorescence indicates porphyrin-producing Gram-positive and Gram-negative bacteria, while cyan fluorescence is associated with Pseudomonas aeruginosa due to the presence of pyoverdines. The FDA has cleared the MolecuLight device to detect Pseudomonas aeruginosa specifically, recognizing its diagnostic value in identifying this common and often resistant pathogen in wounds. This regulatory clearance reinforces the device's credibility and underscores its utility in guiding targeted interventions against Pseudomonas-related infections.
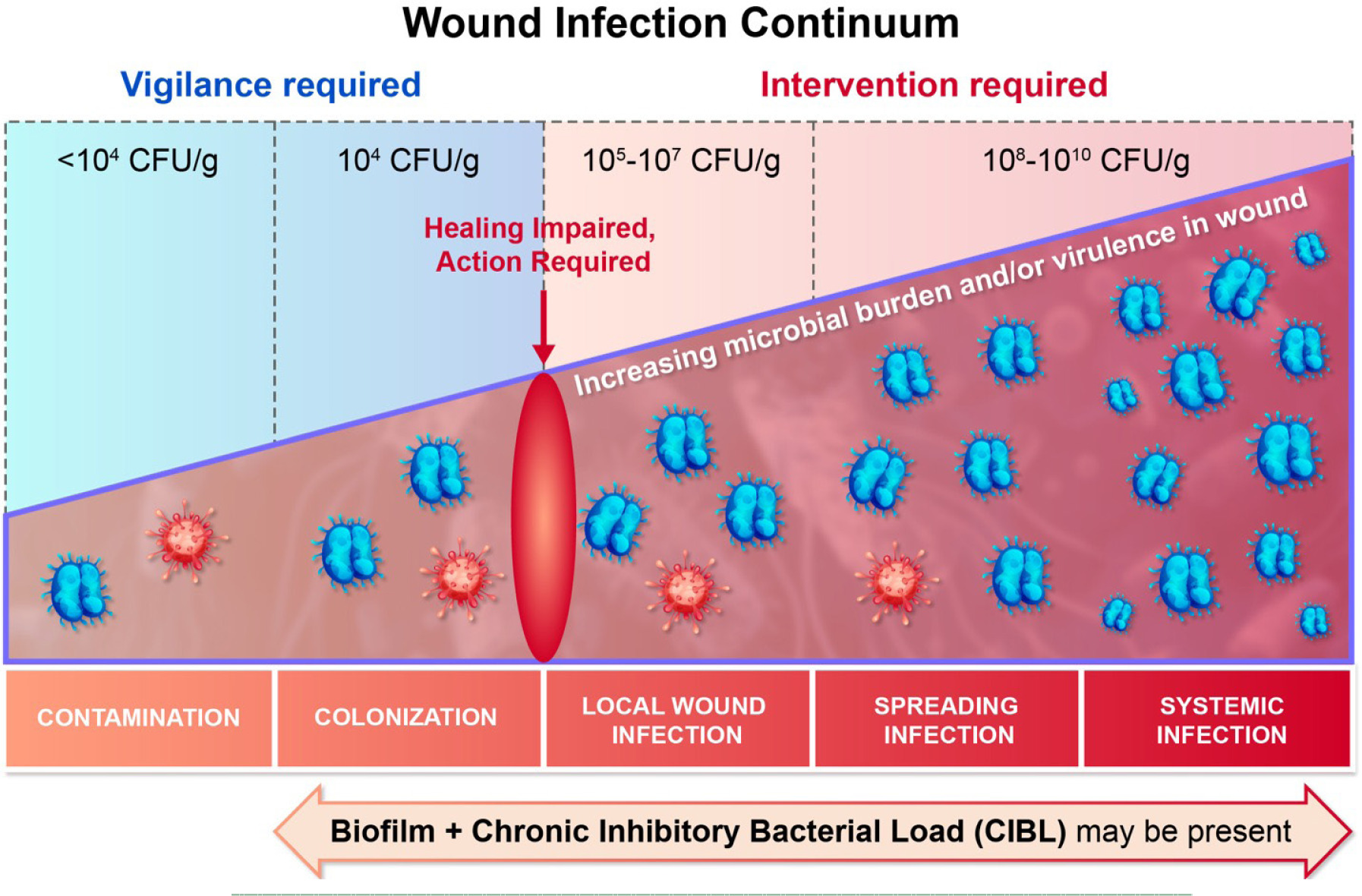
The device immediately visualizes chronic inhibitory bacterial loads (CIBL). CIBL is defined as the persistent presence of bacteria in a wound or its surrounding tissue at concentrations which can inhibit healing, as well as require clinical intervention, with or without the presence of clinical symptoms.3 It refers to the level of bacteria in a nonhealing wound that impedes wound healing.4 This has previously been referred to as critical colonization. Figure 1 depicts the wound infection continuum.
FIGURE 1 The wound infection continuum.
The use of fluorescence imaging allows the clinician to detect both surface and subsurface bacterial burden, including planktonic and biofilm-associated organisms, with high sensitivity and specificity. Notably, this imaging does not require contrast agents or dyes, making it safe for repeated use in diverse clinical environments. However, it does require near absolute darkness. Additionally, the device is compact and handheld, enabling point-of-care application directly at the bedside, in outpatient wound clinics, and intra-operatively in the surgical suite. A sterile sleeve is available for surgeries requiring a sterile field.
The fluorescence imaging occurs in real time and the images are immediately available for interpretation and can be stored digitally for documentation, longitudinal monitoring, and patient education. This immediacy supports real-time decision-making, which is particularly valuable in time-sensitive or resource-constrained settings. Moreover, by facilitating earlier identification of clinically significant bacterial loads, fluorescence imaging can enable proactive treatment interventions that reduce complications and enhance healing trajectories.5
The ability to immediately visualize bacterial burden not only informs clinical decision-making but also empowers practitioners to tailor their surgical strategy and periwound skin management based on the visualization of the microbial contamination. This includes decisions around targeted debridement, graft placement, timing of wound closure, antibiotic stewardship, and post-operative care. Integrating fluorescence imaging into the surgical workflow enhances accuracy, reduces variability between clinicians, and introduces an objective standard that complements traditional wound assessment tools.6
Beyond clinical benefits, fluorescence imaging carries implications for healthcare systems and patient outcomes. By enabling earlier and more accurate detection of the presence of bacteria (>104), it may help reduce the frequency of reoperations, hospital readmissions, prolonged antibiotic use, and the associated costs of delayed wound healing.7
Moreover, it aligns with ongoing initiatives in antimicrobial stewardship and value-based care by potentially reducing unnecessary interventions.
Method of consensus development
To explore the clinical utility of fluorescence imaging in surgical practice, a 2-hour roundtable focused discussion was conducted in March 2025 in Fort Lauderdale, Florida, comprising of 8 panelists: 3 general surgeons, 2 plastic surgeons, 2 wound care specialists, and 1 infectious disease expert, practicing across North America. Participants received pre-meeting literature summaries and usage case studies. The session was chaired by Dr. Thomas Serena, convened and recorded, with thematic analysis used to extract consensus points. The panel evaluated current evidence, shared clinical experiences, and formulated consensus recommendations. Consensus was defined as ≥80% agreement on each point discussed.
This document presents the findings and recommendations of that panel, focusing on the fluorescence imaging integration during the three critical phases of surgical care: pre-operative planning, intra-operative assessment, and post-operative surveillance.
Pre-operative applications
Consensus Statement 1: Fluorescence imaging should be incorporated into pre-operative assessment to objectively identify wounds with elevated bacterial burden, enabling risk stratification, tailored protocol planning, patient education, and interdisciplinary collaboration.
Fluorescence imaging during the pre-operative phase allows for the objective assessment of wound bed readiness and identification of areas requiring targeted debridement. In one study, clinicians using the MolecuLight device achieved >94% accuracy in wound measurements, enabling effective treatment documentation and supporting reimbursement practices.5
Baseline fluorescence imaging provides clinicians with critical visual information to determine the presence and extent of bacterial burden before initiating surgical interventions. This helps stratify patient risk and select appropriate preoperative protocols, such as extended antimicrobial preparation or adjusted debridement strategies. Establishing bacterial mapping at this stage also allows surgeons to track microbial changes throughout the continuum of care, ensuring precise targeting of infected tissues.
In procedures involving grafts or biologics, fluorescence imaging informs the timing and readiness of the wound bed. The device’s ability to visualize infection-prone zones allows clinicians to preemptively address microbial colonization, thereby improving engraftment success and reducing biomaterial failure. A retrospective study of foot ulcers estimated that the implementation of fluorescence imaging increased clinic throughput, reduced antimicrobial dressing expenditures and antibiotic prescribing, and improved healing rates within 12 weeks. Collectively, these changes contributed to a projected 10% reduction in annual wound care costs.8 Additionally, pressure ulcers evaluated with fluorescence imaging healed 27.7% faster compared to standard care (4.8 weeks vs. 17.2 weeks).9 Furthermore, a large randomized controlled trial on MolecuLight demonstrated significantly improved clinical outcomes, such as reduced time to healing and reduced length of stay, in diabetic foot ulcers.10
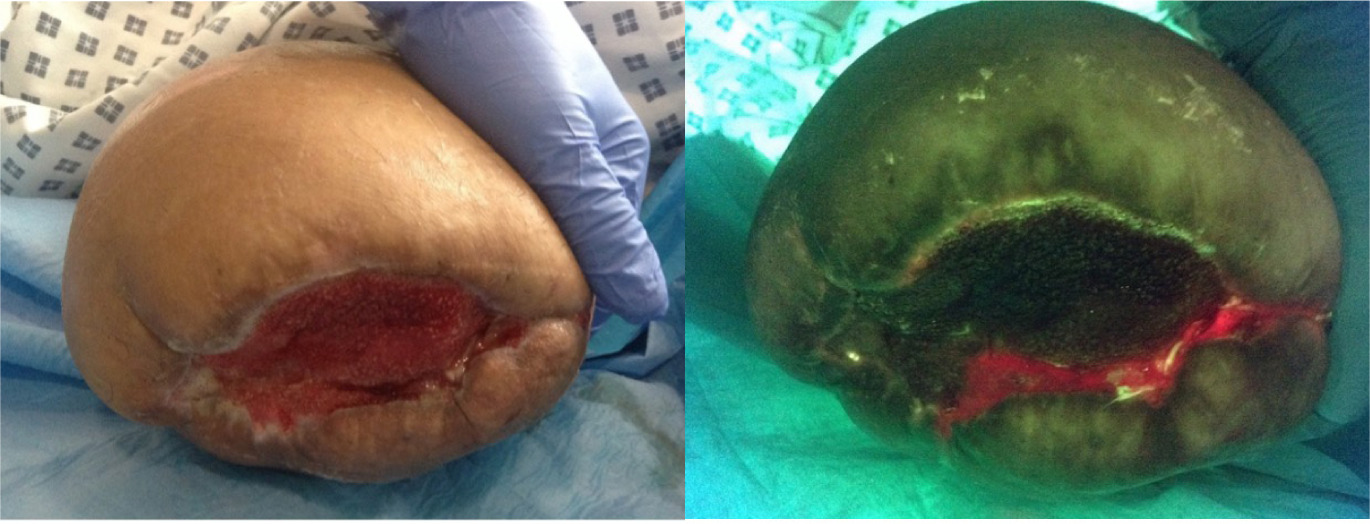
Fluorescence imaging also assists in pre-surgical consultations and patient education. By visually demonstrating bacterial load and wound progression, clinicians can better communicate the need for specific interventions, set realistic expectations, and improve adherence to perioperative care plans. This visual feedback is particularly valuable in high-risk populations, such as those with diabetes or peripheral arterial disease, where wound healing is often compromised. Figure 2 highlights pre-operative use of the device.
FIGURE 2 A patient was due to have skin grafting, but following the use of the visualizing device, this was halted due to bacterial presence and a failed operation was prevented.11
Additionally, imaging data supports interdisciplinary coordination between wound care specialists, surgeons, and infection control teams. Standardized visual assessments can streamline referral decisions, enhance surgical planning, and inform decisions regarding prophylactic antibiotic use. In cases requiring serial surgical staging, such as in extensive debridement or flap reconstructions, fluorescence imaging helps define operative timelines and benchmarks for wound readiness.
Intra-operative applications
Consensus Statement 2: Real-time use of fluorescence imaging intra-operatively is recommended to confirm an aseptic surgical field and subsequently to improve debridement accuracy, surgical and graft outcomes.
In the operating theatre, fluorescence imaging serves as a real-time guide for precision debridement and ensures bacterial burden is adequately reduced before wound closure. This intra-operative use addresses one of the most critical stages of infection prevention—confirmation that the wound bed is as aseptic as possible prior to surgical closure or graft application. High bacterial load, particularly biofilm-embedded pathogens, has been directly correlated with delayed healing, graft failure, and surgical site infections (SSIs).10
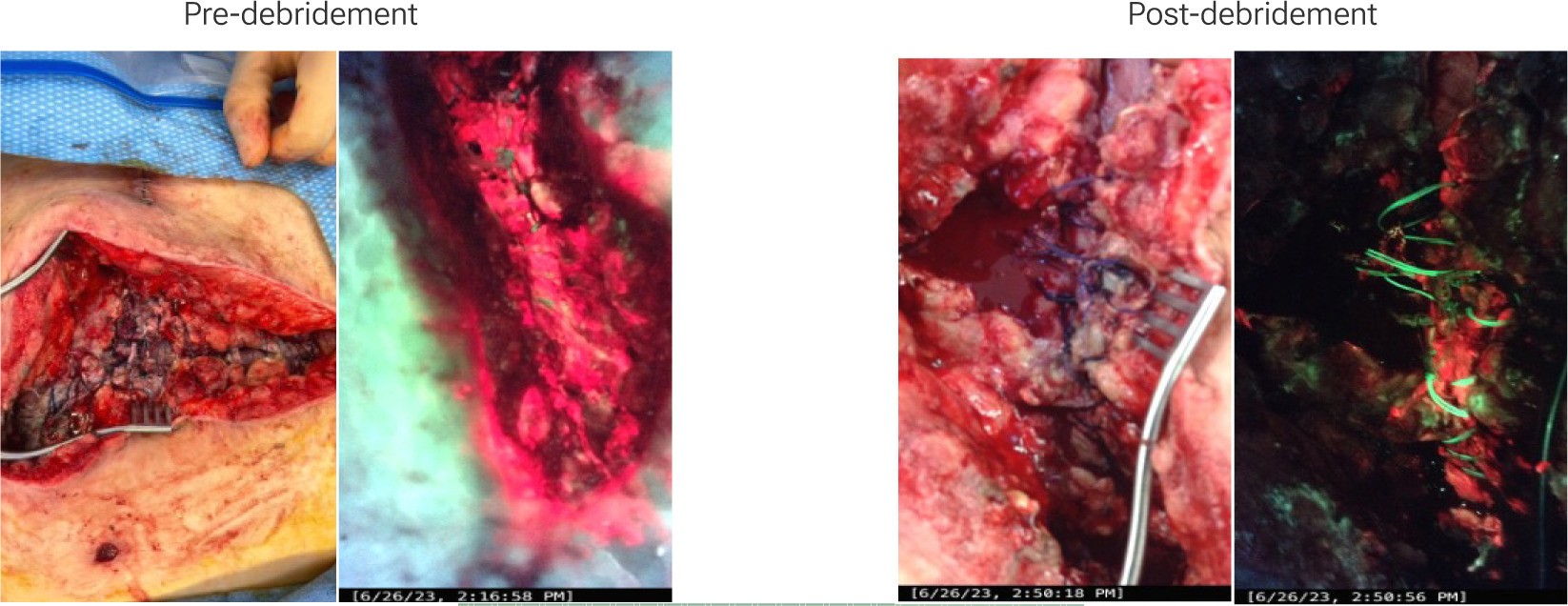
Fluorescence imaging enables visualization of bacterial “hotspots” that may otherwise appear benign under ambient light. Surgeons can visualize fluorescence signals during surgery and tailor debridement to include these regions. The ability to assess the success of debridement in real time can significantly reduce the need for revision surgeries (Figure 3). In one study, fluorescence imaging showed a 94% predictive accuracy for graft outcomes in burn patients, guiding surgeons on optimal grafting sites.11
FIGURE 3 Fluorescence revealed extensive bacteria in a dehisced abdominal wound following ileostomy take down procedure. Targeted excisional debridement in the operating room under fluorescence imaging effectively reduced bacterial colonization. (Images provided courtesy of Dr. Misael Chaen Alonso, MD FACP CWSP FAPWCA)
In reconstructive surgeries, particularly in flap and graft procedures, intra-operative fluorescence imaging plays a vital role in improving the quality and longevity of tissue integration. It aids in reducing the bacterial burden that threatens flap viability or may compromise suture lines. This technology is also helpful in complex procedures such as limb salvage surgeries where precision debridement may determine limb preservation outcomes.
In orthopedic operations, including open fracture fixation and prosthetic joint implantation, intra-operative imaging helps prevent periprosthetic joint infections by identifying microbial contamination around bone and implant surfaces.
Similarly, in cardiac procedures involving sternal wounds or device implantation, such as pacemaker or LVAD placements, the device provides a non-invasive yet sensitive way to ensure the operative field is sufficiently disinfected. The device’s value also extends to intra-abdominal and perineal procedures,12 where the confined anatomy increases the risk of missed contamination. Fluorescence imaging can be adapted to laparotomy settings to identify bacterial contamination before closing deeper anatomical planes. In future iterations, laparoscopic-compatible fluorescence devices may open the door to intra-operative bacterial visualization during minimally invasive surgeries.
It must be noted, that unless an institutional protocol is developed it is quite difficult to to sufficiently darken the operating room to use the device. Therefore, there is a need to use the dark drape that is available, distinct from the sterile sleeve. The sterile sleeve goes over the device to maintain an aseptic field, and the dark drape attaches to the device to enable darkness in the imaging field.
Post-operative surveillance
Consensus Statement 3: Post-operative fluorescence imaging at early follow-up (1-2 weeks) enables timely detection of subclinical bacterial burden, facilitates monitoring of therapeutic response, and supports escalation protocols thereby enhancing early intervention and reducing the risk of surgical site infections.
Post-operatively, fluorescence imaging is employed to monitor wound healing and detect subclinical bacterial growth before visible symptoms manifest. This surveillance function is especially valuable in the first 7–14 days following surgery, a critical window when microbial proliferation can compromise wound integrity. In one retrospective analysis, patients with deep sternal wound infections managed with fluorescence-guided care exhibited reduced ICU length of stay and fewer reinfections.13
Monitoring bacterial status post-closure helps to guide dressing changes, assess the efficacy of topical or systemic antimicrobials, and optimize wound management protocols. The ability to visualize bacterial recurrence non-invasively minimizes unnecessary interventions, supports evidence-based continuation or discontinuation of antibiotics, and enhances clinician confidence in wound healing trajectories.
In high-risk populations, such as diabetic, immunocompromised, and vascular-compromised patients, post-operative surveillance with fluorescence imaging has the potential to pre-empt wound dehiscence, cellulitis, and deep tissue infection. For example, in transplant patients with surgical wounds near vascular anastomoses, early bacterial detection reduces the risk of systemic infection and graft rejection. Further research is underway to formally evaluate the use of fluorescence imaging in these populations.
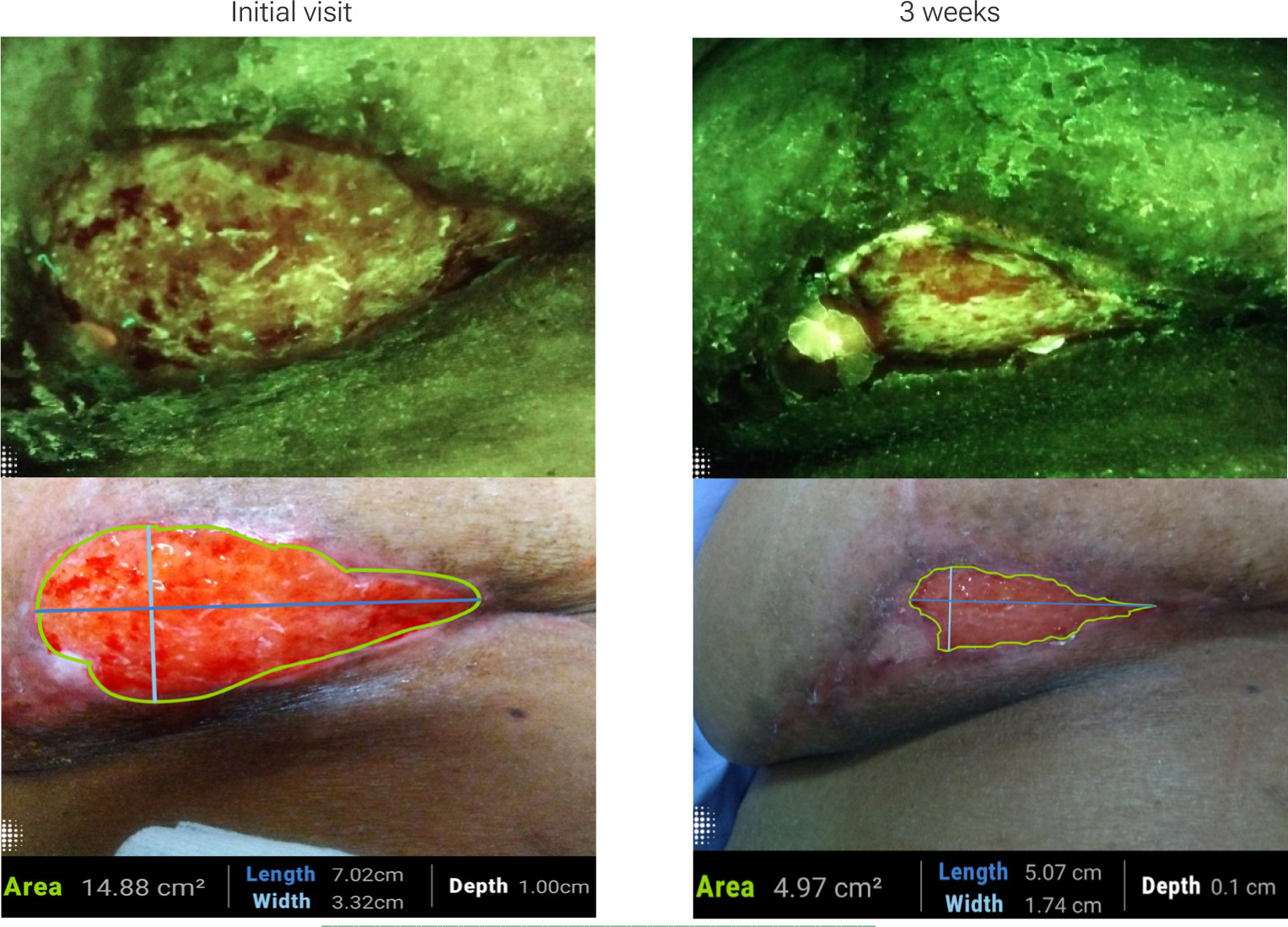
Repeated imaging also supports comparative evaluations during follow-up visits, helping track wound progression objectively (Figure 4). These visual records can be shared across care teams, including home health and outpatient services, facilitating continuity of care. Additionally, they provide tangible reassurance to patients who can see improvements and better understand the healing process, which may improve their adherence to aftercare instructions.
FIGURE 4 Red fluorescence signals decrease alongside improvements in wound closure following breast reduction surgery (Images provided courtesy of Moleculight).
Bacterial surveillance also has medico-legal implications. Visual documentation of a clean post-operative wound may protect clinicians in the event of litigation by establishing baseline conditions. Such documentation serves as a verifiable record of the wound's bacterial status at the time of surgical closure or early post-operative follow-up. In cases where complications arise later, the availability of high-quality, time-stamped images may demonstrate that the standard of care was met at critical points. Likewise, prompt identification of infection, documented through fluorescence imaging, can reinforce that clinicians responded appropriately and in a timely manner, fulfilling professional and regulatory responsibilities. This not only protects clinicians legally but also enhances institutional transparency and patient trust.
Moreover, these records may be valuable in quality assurance initiatives and institutional reviews, serving as educational tools and evidence for refining clinical protocols. As healthcare systems increasingly emphasize outcome-based care and data-driven accountability, the integration of fluorescence imaging into routine post-operative surveillance offers both protective and progressive value.
Fluorescence imaging and antibiotic stewardship
The overuse of antibiotics in chronic wound care has escalated antimicrobial resistance.14,15 Fluorescence imaging helps differentiate wounds that require antimicrobial intervention from those that do not. One study showed a shift towards topical approaches over systemic.15 Another study demonstrated a 33% reduction in antibiotic prescriptions and a 49% reduction in antimicrobial dressing use following the integration of fluorescence imaging.8 This approach aligns with antimicrobial stewardship principles while improving 12-week healing outcomes. This requires the clinician to adhere to the clinical data provided by the device. By visualizing CIBL, clinicians can confidently pursue hygiene-based or targeted antimicrobial strategies as appropriate.
Applications across surgical specialties
Fluorescence imaging has been used with promising outcomes in transplant, plastic, cardiovascular, and obstetric surgeries:
-
In one study of kidney transplant recipients with organ/space SSI, the integration of real-time fluorescence imaging with negative pressure wound therapy (NPWT) achieved infection control and preserved graft function.16
-
In a single site study of deep sternal wound infection (DSWI), fluorescence-guided debridement significantly improved infection resolution and clinical outcomes, with patients experiencing reduced reinfection and osteomyelitis recurrence.7
-
Fluorescence imaging used during a prospective cohort study of obstetric patients who incurred a perineal infection yielded an 80% sensitivity for clinically significant bacterial burden.17
-
In plastic and reconstructive surgery, particularly in complex flap reconstructions, fluorescence imaging identifies residual bacterial contamination that could jeopardize flap viability. Surgeons are able to debride targeted areas before performing microvascular anastomoses, thus improving flap success rates.
-
In orthopedic surgeries, fluorescence imaging has been proposed for monitoring prosthetic joint surgeries or large wound reconstructions, allowing surgeons to detect bacterial load that could contribute to post-operative prosthetic joint infections (PJIs). Early detection in such cases may help preserve the implant and avoid more invasive re-interventions.
-
In colorectal and general surgeries, fluorescence imaging could be explored in assessment of abdominal or perineal wound contamination post-operatively, particularly in patients with a stoma or surgical drains. Imaging enables the early identification of bacterial recolonization in incisional sites and helps dictate dressing changes or antibiotic application.
-
In breast surgeries, particularly mastectomy and reconstructive procedures, fluorescence imaging may support early wound bed evaluation, enabling oncological surgeons to reduce complications such as flap necrosis and SSIs in high-risk patients receiving chemotherapy or radiation.
These applications illustrate the potential for the use of fluorescence imaging across surgical disciplines, and its promising role in improving outcomes, minimizing complications, and informing post-operative management.
Future considerations
The panel advocated for expanded clinical implementation of fluorescence imaging and development of laparoscopic-compatible models. Further studies are needed to evaluate outcomes stratified by comorbidities and to establish best practices across surgical settings. A follow-up consensus meeting is planned to review adoption trends and clinical impact.
Conclusion
The panel reached a consensus that fluorescence imaging is a technology that has the potential to improve surgical wound care by introducing objective, real-time bacterial visualization throughout the perioperative continuum (Table 1). Pre-operatively, it may assist with treatment planning, patient education, and interdisciplinary collaboration. Intra-operatively, it has the potential to improve debridement accuracy and graft outcomes, while post-operatively, it enables ongoing monitoring to pre-empt complications. This potentially could reduce the incidence of SSIs, enhancing surgical precision, and supporting antimicrobial stewardship. Fluorescence imaging may address some of the key challenges in modern surgical practice.
TABLE 1 Summary of consensus statements
| Statement 1 | Fluorescence imaging should be incorporated into pre-operative assessment to objectively identify wounds with elevated bacterial burden, enabling risk stratification, tailored protocol planning, patient education, and interdisciplinary collaboration. |
| Statement 2 | Real-time use of fluorescence imaging intra-operatively is recommended to confirm an aseptic surgical field and subsequently to improve debridement accuracy, surgical and graft outcomes. |
| Statement 3 | Post-operative fluorescence imaging at early follow-up (1-2 weeks) enables timely detection of subclinical bacterial burden, facilitates monitoring of therapeutic response, and supports escalation protocols thereby enhancing early intervention and reducing the risk of surgical site infections. |
The authors anticipate more specialties may integrate fluorescence imaging into their workflows, and the evidence base will grow, strengthening its role as a standard adjunct in surgical management. Future developments, including laparoscopic and robotic-compatible imaging platforms, will further expand its utility in minimally invasive and complex surgical procedures. Given its cost-effectiveness, ease of use, and growing body of supportive evidence, the MolecuLight imaging device and similar tools are poised to redefine best practices in surgical wound care.